Radical (chemistry)

 Clash Royale CLAN TAG#URR8PPP
Clash Royale CLAN TAG#URR8PPP

The hydroxyl radical, Lewis structure shown, contains one unpaired electron
In chemistry, a radical is an atom, molecule, or ion that has an unpaired valence electron.[1][2]
With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes.
A notable example of a radical is the hydroxyl radical (HO•), a molecule that has one unpaired electron on the oxygen atom. Two other examples are triplet oxygen and triplet carbene (:CH
2) which have two unpaired electrons.
Radicals may be generated in a number of ways, but typical methods involve redox reactions. Ionizing radiation, heat, electrical discharges, and electrolysis are known to produce radicals. Radicals are intermediates in many chemical reactions, more so than is apparent from the balanced equations.
Radicals are important in combustion, atmospheric chemistry, polymerization, plasma chemistry, biochemistry, and many other chemical processes. A large fraction of natural products is generated by radical-generating enzymes. In living organisms, the radicals superoxide and nitric oxide and their reaction products regulate many processes, such as control of vascular tone and thus blood pressure. They also play a key role in the intermediary metabolism of various biological compounds. Such radicals can even be messengers in a process dubbed redox signaling. A radical may be trapped within a solvent cage or be otherwise bound.
Contents
1 Depiction in chemical reactions
2 Formation
2.1 Homolysis
2.2 From other radicals
2.3 One electron redox
3 Persistence and stability
3.1 Stable radicals
3.2 Persistent radicals
3.3 Diradicals
4 Reactivity
5 Combustion
6 Polymerization
7 Atmospheric radicals
8 In biology
8.1 Reactive oxygen species
9 History and nomenclature
10 Diagnostics
11 See also
12 References
Depiction in chemical reactions
In chemical equations, radicals are frequently denoted by a dot placed immediately to the right of the atomic symbol or molecular formula as follows:
- Cl2→UV2Cl⋅displaystyle mathrm Cl _2;xrightarrow UV;2mathrm Cl cdot
Radical reaction mechanisms use single-headed arrows to depict the movement of single electrons:
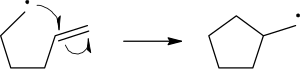
The homolytic cleavage of the breaking bond is drawn with a 'fish-hook' arrow to distinguish from the usual movement of two electrons depicted by a standard curly arrow. The second electron of the breaking bond also moves to pair up with the attacking radical electron; this is not explicitly indicated in this case.
Radicals also take part in radical addition and radical substitution as reactive intermediates. Chain reactions involving radicals can usually be divided into three distinct processes. These are initiation, propagation, and termination.
Initiation reactions are those that result in a net increase in the number of radicals. They may involve the formation of radicals from stable species as in Reaction 1 above or they may involve reactions of radicals with stable species to form more radicals.
Propagation reactions are those reactions involving radicals in which the total number of radicals remains the same.
Termination reactions are those reactions resulting in a net decrease in the number of radicals. Typically two radicals combine to form a more stable species, for example: 2Cl·→ Cl2
Formation
Homolysis
Radicals can form by breaking of covalent bonds by homolysis. The homolytic bond dissociation energies, usually abbreviated as "ΔH °" are a measure of bond strength. Splitting H2 into 2H•, for example, requires a ΔH ° of +435 kJ·mol-1, while splitting Cl2 into 2Cl• requires a ΔH ° of +243 kJ·mol-1. For weak bonds, homolysis can be induced thermally. Strong bonds require high energy photons or even flames to induce homolysis.
From other radicals
Radicals or charged species add to non-radicals to give new radicals. This process is the basis of the radical chain reaction.
Being prevalent and a diradical, O2 reacts with many organic compounds to generate radicals together with the hydroperoxide radical. This process is related to rancidification of unsaturated fats.
One electron redox
Radicals may also be formed by single-electron oxidation or reduction of an atom or molecule. These redox reactions occur in electrochemical cells and in ionization chambers of mass spectrometers.
Persistence and stability

Although radicals are generally short-lived due to their reactivity, there are long-lived radicals. These are categorized as follows:
Stable radicals
The prime example of a stable radical is molecular dioxygen (O2). Another common example is nitric oxide (NO). Organic radicals can be long lived if they occur in a conjugated π system, such as the radical derived from α-tocopherol (vitamin E). There are also hundreds of examples of thiazyl radicals, which show low reactivity and remarkable thermodynamic stability with only a very limited extent of π resonance stabilization.[3][4]
Persistent radicals
Persistent radical compounds are those whose longevity is due to steric crowding around the radical center, which makes it physically difficult for the radical to react with another molecule.[5] Examples of these include Gomberg's triphenylmethyl radical, Fremy's salt (Potassium nitrosodisulfonate, (KSO3)2NO·), aminoxyls, (general formula R2NO·) such as TEMPO, TEMPOL, nitronyl nitroxides, and azephenylenyls and radicals derived from PTM (perchlorophenylmethyl radical) and TTM (tris(2,4,6-trichlorophenyl)methyl radical). Persistent radicals are generated in great quantity during combustion, and "may be responsible for the oxidative stress resulting in cardiopulmonary disease and probably cancer that has been attributed to exposure to airborne fine particles".[6]
Gomberg's free radical can be generated by following reaction in lab -
(Ph)3C-Cl + Ag === (Ph)3C• + AgCl
The reason for persistivity of free radicals is either the delocalisation of unpaired electron ( e.g. triphenylmethyl radical ) or the unavailability of unpaired electron to other species due to the screening of neighbouring atoms/groups ( e.g. tri-tert-butylphenoxyl radical ).
Diradicals
Diradicals are molecules containing two radical centers. Multiple radical centers can exist in a molecule. Atmospheric oxygen naturally exists as a diradical in its ground state as triplet oxygen. The low reactivity of atmospheric oxygen is due to its diradical state. Non-radical states of dioxygen are actually less stable than the diradical. The relative stability of the oxygen diradical is primarily due to the spin-forbidden nature of the triplet-singlet transition required for it to grab electrons, i.e., "oxidize". The diradical state of oxygen also results in its paramagnetic character, which is demonstrated by its attraction to an external magnet.[7] Diradicals can also occur in metal-oxo complexes, lending themselves for studies of spin forbidden reactions in transition metal chemistry.[8]Carbenes in their triplet state can be viewed as diradicals centred on the same atom, while these are usually highly reactive persistent carbenes are known, with N-heterocyclic carbenes being the most common example.
Reactivity
Radical alkyl intermediates are stabilized by similar physical processes to carbocations: as a general rule, the more substituted the radical center is, the more stable it is. This directs their reactions. Thus, formation of a tertiary radical (R3C·) is favored over secondary (R2HC·), which is favored over primary (RH2C·). Likewise, radicals next to functional groups such as carbonyl, nitrile, and ether are more stable than tertiary alkyl radicals.
Radicals attack double bonds. However, unlike similar ions, such radical reactions are not as much directed by electrostatic interactions. For example, the reactivity of nucleophilic ions with α,β-unsaturated compounds (C=C–C=O) is directed by the electron-withdrawing effect of the oxygen, resulting in a partial positive charge on the carbonyl carbon. There are two reactions that are observed in the ionic case: the carbonyl is attacked in a direct addition to carbonyl, or the vinyl is attacked in conjugate addition, and in either case, the charge on the nucleophile is taken by the oxygen. Radicals add rapidly to the double bond, and the resulting α-radical carbonyl is relatively stable; it can couple with another molecule or be oxidized. Nonetheless, the electrophilic/neutrophilic character of radicals has been shown in a variety of instances. One example is the alternating tendency of the copolymerization of maleic anhydride (electrophilic) and styrene (slightly nucleophilic).
In intramolecular reactions, precise control can be achieved despite the extreme reactivity of radicals. In general, radicals attack the closest reactive site the most readily. Therefore, when there is a choice, a preference for five-membered rings is observed: four-membered rings are too strained, and collisions with carbons six or more atoms away in the chain are infrequent.
Triplet carbenes and nitrenes, which are diradicals, have distinctive chemistry.
Combustion
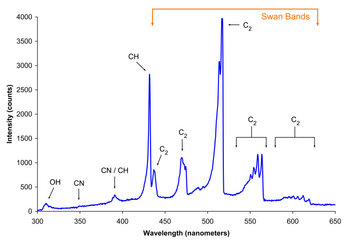
Spectrum of the blue flame from a butane torch showing excited molecular radical band emission and Swan bands
A familiar radical reaction is combustion. The oxygen molecule is a stable diradical, best represented by ·O-O·. Because spins of the electrons are parallel, this molecule is stable. While the ground state of oxygen is this unreactive spin-unpaired (triplet) diradical, an extremely reactive spin-paired (singlet) state is available. For combustion to occur, the energy barrier between these must be overcome. This barrier can be overcome by heat, requiring high temperatures. The triplet-singlet transition is also "forbidden". This presents an additional barrier to the reaction. It also means molecular oxygen is relatively unreactive at room temperature except in the presence of a catalytic heavy atom such as iron or copper.
Combustion consists of various radical chain reactions that the singlet radical can initiate. The flammability of a given material strongly depends on the concentration of radicals that must be obtained before initiation and propagation reactions dominate leading to combustion of the material. Once the combustible material has been consumed, termination reactions again dominate and the flame dies out. As indicated, promotion of propagation or termination reactions alters flammability. For example, because lead itself deactivates radicals in the gasoline-air mixture, tetraethyl lead was once commonly added to gasoline. This prevents the combustion from initiating in an uncontrolled manner or in unburnt residues (engine knocking) or premature ignition (preignition).
When a hydrocarbon is burned, a large number of different oxygen radicals are involved. Initially, hydroperoxyl radical (HOO·) are formed. These then react further to give organic hydroperoxides that break up into hydroxyl radicals (HO·).
Polymerization
In addition to combustion, many polymerization reactions involve radicals. As a result, many plastics, enamels, and other polymers are formed through radical polymerization. For instance, drying oils and alkyd paints harden due to radical crosslinking by oxygen from the atmosphere.
Recent advances in radical polymerization methods, known as living radical polymerization, include:
- Reversible addition-fragmentation chain transfer (RAFT)
- Atom transfer radical polymerization (ATRP)
- Nitroxide mediated polymerization (NMP)
These methods produce polymers with a much narrower distribution of molecular weights.
Atmospheric radicals
The most common radical in the lower atmosphere is molecular dioxygen. Photodissociation of source molecules produces other radicals. In the lower atmosphere, important radical are produced by the photodissociation of nitrogen dioxide to an oxygen atom and nitric oxide (see eq. 1. 1 below), which plays a key role in smog formation—and the photodissociation of ozone to give the excited oxygen atom O(1D) (see eq. 1. 2 below). The net and return reactions are also shown (eq. 1. 3 and eq. 1. 4, respectively).
NO2→hνNO+Odisplaystyle ce NO2->[h nu] NO+ O
(eq. 1. 1)
O+O2⟶O3displaystyle ce O+ O2-> O3
(eq. 1. 2)
NO2+O2→hνNO+O3displaystyle ce NO2+ O2->[h nu] NO+ O3
(eq. 1. 3)
NO+O3⟶NO2+O2displaystyle ce NO+ O3-> NO2+ O2
(eq. 1. 4)
In the upper atmosphere, the photodissociation of normally unreactive chlorofluorocarbons (CFCs) by solar ultraviolet radiation is an important source of radicals (see eq. 1 below). These reactions give the chlorine radical, Cl•, which catalyzes the conversion of ozone to O2, i.e., Ozone depletion (eq. 2. 2–eq. 2. 4 below).
CFCS→hνCl⋅displaystyle ce CFCS->[h nu] Cl.
(eq. 2. 1)
Cl⋅+O3⟶ClO⋅+O2displaystyle ce Cl.+ O3-> ClO.+ O2
(eq. 2. 2)
O3→hνO+O2displaystyle ce O3->[h nu] O+ O2
(eq. 2. 3)
O+ClO⋅⟶Cl⋅+O2displaystyle ce O+ ClO.-> Cl.+ O2
(eq. 2. 4)
2O3→hν3O2displaystyle ce 2O3->[h nu] 3O2
(eq. 2. 5)
Such reactions cause the depletion of the ozone layer, especially since the chlorine radical is free to engage in another reaction chain; consequently, the use of chlorofluorocarbons as refrigerants has been restricted.
In biology
Radicals play important roles in biology. Many of these are necessary for life, such as the intracellular killing of bacteria by phagocytic cells such as granulocytes and macrophages. Radicals are involved in cell signalling processes,[9] known as redox signaling. For example, radical attack of linoleic acid produces a series of 13-Hydroxyoctadecadienoic acids and 9-Hydroxyoctadecadienoic acids, which may act to regulate localized tissue inflammatory and/or healing responses, pain perception, and the proliferation of malignant cells. Radical attacks on arachidonic acid and docosahexaenoic acid produce a similar but broader array of signaling products.[10]

Structure of the deoxyadenosyl radical, a common biosynthetic intermediate.[11]
Radicals may also be involved in Parkinson's disease, senile and drug-induced deafness, schizophrenia, and Alzheimer's.[12] The classic free-radical syndrome, the iron-storage disease hemochromatosis, is typically associated with a constellation of free-radical-related symptoms including movement disorder, psychosis, skin pigmentary melanin abnormalities, deafness, arthritis, and diabetes mellitus. The free-radical theory of aging proposes that radicals underlie the aging process itself. Similarly, the process of mitohormesis suggests that repeated exposure to radicals may extend life span.
Because radicals are necessary for life, the body has a number of mechanisms to minimize radical-induced damage and to repair damage that occurs, such as the enzymes superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase. In addition, antioxidants play a key role in these defense mechanisms. These are often the three vitamins, vitamin A, vitamin C and vitamin E and polyphenol antioxidants. Furthermore, there is good evidence indicating that bilirubin and uric acid can act as antioxidants to help neutralize certain radicals. Bilirubin comes from the breakdown of red blood cells' contents, while uric acid is a breakdown product of purines. Too much bilirubin, though, can lead to jaundice, which could eventually damage the central nervous system, while too much uric acid causes gout.[13]
Reactive oxygen species
Reactive oxygen species or ROS are species such as superoxide, hydrogen peroxide, and hydroxyl radical, commonly associated with cell damage. ROS form as a natural by-product of the normal metabolism of oxygen and have important roles in cell signaling.
Two important oxygen-centered radicals are superoxide and hydroxyl radical. They derive from molecular oxygen under reducing conditions. However, because of their reactivity, these same radicals can participate in unwanted side reactions resulting in cell damage. Excessive amounts of these radicals can lead to cell injury and death, which may contribute to many diseases such as cancer, stroke, myocardial infarction, diabetes and major disorders.[14] Many forms of cancer are thought to be the result of reactions between radicals and DNA, potentially resulting in mutations that can adversely affect the cell cycle and potentially lead to malignancy.[15] Some of the symptoms of aging such as atherosclerosis are also attributed to radical induced oxidation of cholesterol to 7-ketocholesterol.[16] In addition radicals contribute to alcohol-induced liver damage, perhaps more than alcohol itself. Radicals produced by cigarette smoke are implicated in inactivation of alpha 1-antitrypsin in the lung. This process promotes the development of emphysema.
Oxybenzone has been found to form radicals in sunlight, and therefore may be associated with cell damage as well. This only occurred when it was combined with other ingredients commonly found in sunscreens, like titanium oxide and octyl methoxycinnamate.[17]
ROS attack the polyunsaturated fatty acid, linoleic acid, to form a series of 13-Hydroxyoctadecadienoic acid and 9-Hydroxyoctadecadienoic acid products that serve as signaling molecules that may trigger responses that counter the tissue injury which caused their formation. ROS attacks other polyunsaturated fatty acids, e.g. arachidonic acid and docosahexaenoic acid, to produce a similar series of signaling products.[18]
History and nomenclature

Moses Gomberg (1866–1947), the founder of radical chemistry
Until late in the 20th century the word "radical" was used in chemistry to indicate any connected group of atoms, such as a methyl group or a carboxyl, whether it was part of a larger molecule or a molecule on its own. The qualifier "free" was then needed to specify the unbound case. Following recent nomenclature revisions, a part of a larger molecule is now called a functional group or substituent, and "radical" now implies "free". However, the old nomenclature may still appear in some books.
The term radical was already in use when the now obsolete radical theory was developed. Louis-Bernard Guyton de Morveau introduced the phrase "radical" in 1785 and the phrase was employed by Antoine Lavoisier in 1789 in his Traité Élémentaire de Chimie. A radical was then identified as the root base of certain acids (the Latin word "radix" meaning "root"). Historically, the term radical in radical theory was also used for bound parts of the molecule, especially when they remain unchanged in reactions. These are now called functional groups. For example, methyl alcohol was described as consisting of a methyl "radical" and a hydroxyl "radical". Neither are radicals in the modern chemical sense, as they are permanently bound to each other, and have no unpaired, reactive electrons; however, they can be observed as radicals in mass spectrometry when broken apart by irradiation with energetic electrons.
In a modern context the first organic (carbon–containing) radical identified was triphenylmethyl radical, (C6H5)3C•. This species was discovered by Moses Gomberg in 1900. In 1933 Morris Kharash and Frank Mayo proposed that free radicals were responsible for anti-Markovnikov addition of hydrogen bromide to allyl bromide.[19][20]
In most fields of chemistry, the historical definition of radicals contends that the molecules have nonzero electron spin. However, in fields including spectroscopy, chemical reaction, and astrochemistry, the definition is slightly different. Gerhard Herzberg, who won the Nobel prize for his research into the electron structure and geometry of radicals, suggested a looser definition of free radicals: "any transient (chemically unstable) species (atom, molecule, or ion)".[21] The main point of his suggestion is that there are many chemically unstable molecules that have zero spin, such as C2, C3, CH2 and so on. This definition is more convenient for discussions of transient chemical processes and astrochemistry; therefore researchers in these fields prefer to use this loose definition.[22]
Diagnostics
Radicals typically exhibit paramagnetism, but the bulk magnetic properties of an ion or molecule are often not conveniently measured. Electron spin resonance is instead the definitive and most widely used technique for characterizing radicals. The nature of the atom bearing the unpaired electron and its neighboring atoms can often be deduced by the EPR spectrum.[23]
The presence of radicals can also be detected or inferred by chemical reagents that trap (i.e. combine with) radicals. Often these traps are themselves radicals, such as TEMPO.
See also
- Electron pair
- Globally Harmonized System of Classification and Labelling of Chemicals
- Hofmann–Löffler reaction
- Free radical research
- ARC Centre of Excellence for Free Radical Chemistry and Biotechnology
References
^ IUPAC Gold Book radical (free radical) PDF
^ Hayyan, M.; Hashim, M.A.; AlNashef, I.M. (2016). "Superoxide Ion: Generation and Chemical Implications". Chem. Rev. 116 (5): 3029–85. doi:10.1021/acs.chemrev.5b00407. PMID 26875845..mw-parser-output cite.citationfont-style:inherit.mw-parser-output .citation qquotes:"""""""'""'".mw-parser-output .citation .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-ws-icon abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center.mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-maintdisplay:none;color:#33aa33;margin-left:0.3em.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ Oakley, Richard T. (1988). "Cyclic and Heterocyclic Thiazenes" (PDF). Progress in Inorganic Chemistry. Cyclic and Heterocyclic Thiazenes (section). Progress in Inorganic Chemistry. 36. pp. 299–391. doi:10.1002/9780470166376.ch4. ISBN 978-0-470-16637-6.
^ Rawson, J; Banister, A; Lavender, I (1995). Advances in Heterocyclic Chemistry. The Chemistry of Dithiadiazolylium and Dithiadiazolyl Rings (section) =. Advances in Heterocyclic Chemistry. 62. pp. 137–247. doi:10.1016/S0065-2725(08)60422-5. ISBN 978-0-12-020762-6.
^ Griller, David; Ingold, Keith U. (1976). "Persistent carbon-centered radicals". Accounts of Chemical Research. 9: 13–19. doi:10.1021/ar50097a003.
^ Lomnicki S.; Truong H.; Vejerano E.; Dellinger B. (2008). "Copper oxide-based model of persistent free radical formation on combustion-derived particulate matter". Environ. Sci. Technol. 42 (13): 4982–88. Bibcode:2008EnST...42.4982L. doi:10.1021/es071708h. PMID 18678037.
^ However, paramagnetism does not necessarily imply radical character.
^ Linde, C.; Åkermark, B.; Norrby, P.-O.; Svensson, M. (1999). "Timing is Critical: Effect of Spin Changes on the Diastereoselectivity in Mn(Salen)-Catalyzed Epoxidation". Journal of the American Chemical Society. 121 (21): 5083–84. doi:10.1021/ja9809915.
^ Pacher P, Beckman JS, Liaudet L (2007). "Nitric oxide and peroxynitrite in health and disease". Physiol. Rev. 87 (1): 315–424. doi:10.1152/physrev.00029.2006. PMC 2248324. PMID 17237348.
^ Njie-Mbye, Ya Fatou; Kulkarni-Chitnis, Madhura; Opere, Catherine A.; Barrett, Aaron; Ohia, Sunny E. (2013). "Lipid peroxidation: pathophysiological and pharmacological implications in the eye". Frontiers in Physiology. 4: 366. doi:10.3389/fphys.2013.00366. PMC 3863722. PMID 24379787.
^ Broderick, J.B.; Duffus, B.R.; Duschene, K.S.; Shepard, E.M. (2014). "Radical S-Adenosylmethionine Enzymes". Chemical Reviews. 114 (8): 4229–317. doi:10.1021/cr4004709. PMID 24476342.CS1 maint: Uses authors parameter (link)
^ Floyd, R.A. (1999). "Neuroinflammatory processes are important in neurodegenerative diseases: An hypothesis to explain the increased formation of reactive oxygen and nitrogen species as major factors involved in neurodegenerative disease development". Free Radical Biology and Medicine. 26 (9–10): 1346–55. doi:10.1016/s0891-5849(98)00293-7.
^ An overview of the role of radicals in biology and of the use of electron spin resonance in their detection may be found in Rhodes C.J. (2000). Toxicology of the Human Environment – the critical role of free radicals. London: Taylor and Francis. ISBN 978-0-7484-0916-7.
^ Rajamani Karthikeyan; Manivasagam T; Anantharaman P; Balasubramanian T; Somasundaram ST (2011). "Chemopreventive effect of Padina boergesenii extracts on ferric nitrilotriacetate (Fe-NTA)-induced oxidative damage in Wistar rats". J. Appl. Phycol. 23 (2): 257–63. doi:10.1007/s10811-010-9564-0.
^ Mukherjee, P.K.; Marcheselli, V.L.; Serhan, C.N.; Bazan, N.G. (2004). "Neuroprotecin D1: A docosahexanoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress". Proceedings of the National Academy of Sciences of the USA. 101 (22): 8491–96. Bibcode:2004PNAS..101.8491M. doi:10.1073/pnas.0402531101. PMC 420421. PMID 15152078.
^ Lyons, MA; Brown, AJ (1999). "7-Ketocholesterol". Int. J. Biochem. Cell Biol. 31 (3–4): 369–75. doi:10.1016/s1357-2725(98)00123-x. PMID 10224662.
^ Serpone, N; Salinaro, A; Emeline, AV; Horikoshi, S; Hidaka, H; Zhao, JC (2002). "An in vitro systematic spectroscopic examination of the photostabilities of a random set of commercial sunscreen lotions and their chemical UVB/UVA active agents". Photochemical & Photobiological Sciences. 1 (12): 970–81. doi:10.1039/b206338g.
^ Njie-Mbye, Ya Fatou; Kulkarni-Chitnis, Madhura; Opere, Catherine A.; Barrett, Aaron; Ohia, Sunny E. (2013). "Lipid peroxidation: pathophysiological and pharmacological implications in the eye". Frontiers in Physiology. 4. doi:10.3389/fphys.2013.00366.
^ The Peroxide Effect in the Addition of Reagents to Unsaturated Compounds. I. The Addition of Hydrogen Bromide to Allyl Bromide M.S. Kharasch, Frank R. Mayo J. Am. Chem. Soc., 1933, 55, pp. 2468–96 doi:10.1021/ja01333a041
^ Radicals: Reactive Intermediates with Translational Potential Ming Yan, Julian C. Lo, Jacob T. Edwards, and Phil S. Baran J. Am. Chem. Soc., 2016, 138 (39), pp. 12692–714 doi:10.1021/jacs.6b08856
^ G. Herzberg (1971), "The spectra and structures of simple free radicals",
ISBN 0-486-65821-X.
^ 28th International Symposium on Free Radicals Archived 2007-07-16 at the Wayback Machine.
^ Chechik, Victor; Carter, Emma; Murphy, Damien (2016). Electron Paramagnetic Resonance. Oxford University Press. ISBN 978-0-19-872760-6.

![displaystyle ce NO2->[h nu] NO+ O](https://wikimedia.org/api/rest_v1/media/math/render/svg/34fed23155c119954fa08461965c09c3d04f2921)

![displaystyle ce NO2+ O2->[h nu] NO+ O3](https://wikimedia.org/api/rest_v1/media/math/render/svg/1e1d3ed7bb48f69dfa453b9727ecbe7863804988)

![displaystyle ce CFCS->[h nu] Cl.](https://wikimedia.org/api/rest_v1/media/math/render/svg/088ca5853b063101511fb5a21520e648c6d87466)

![displaystyle ce O3->[h nu] O+ O2](https://wikimedia.org/api/rest_v1/media/math/render/svg/f2db8df4adfc00e58fc18167823562cdbaa169d8)

![displaystyle ce 2O3->[h nu] 3O2](https://wikimedia.org/api/rest_v1/media/math/render/svg/29b154561607496cd0feb37e02045d192ecbb4e8)