Saponin
Saponin
Jump to navigation
Jump to search
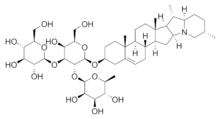
Chemical structure of the saponin solanine
Saponins are a class of chemical compounds found in particular abundance in various plant species. More specifically, they are amphipathic glycosides grouped phenomenologically by the soap-like foam they produce when shaken in aqueous solutions, and structurally by having one or more hydrophilic glycoside moieties combined with a lipophilic triterpene or steroid derivative.[1][2]
Contents
1 Structural variety and biosynthesis
2 Sources
3 Test
4 Role in plant ecology and impact on animal foraging
5 Ethnobotany
6 Research and uses
7 See also
8 References
9 External links
Structural variety and biosynthesis[edit]
The aglycone (glycoside-free) portions of the saponins are termed sapogenins. The number of saccharide chains attached to the sapogenin/aglycone core can vary – giving rise to another dimension of nomenclature (monodesmosidic, bidesmosidic, etc.[1]) – as can the length of each chain. A somewhat dated compilation has the range of saccharide chain lengths being 1–11, with the numbers 2–5 being the most frequent, and with both linear and branched chain saccharides being represented.[1] Dietary monosaccharides such as D-glucose and D-galactose are among the most common components of the attached chains.[1]
The lipophilic aglycone can be any one of a wide variety of polycyclic organic structures originating from the serial addition of 10-carbon (C10) terpene units to compose a C30 triterpene skeleton,[3][4] often with subsequent alteration to produce a C27 steroidal skeleton.[1] The subset of saponins that are steroidal have been termed saraponins.[2] Aglycone derivatives can also incorporate nitrogen, so some saponins also present chemical and pharmacologic characteristics of alkaloid natural products. The figure at right above presents the structure of the alkaloid phytotoxin solanine, a monodesmosidic, branched-saccharide steroidal saponin. (The lipophilic steroidal structure is the series of connected six- and five-membered rings at the right of the structure, while the three oxygen-rich sugar rings are at left and below. Note the nitrogen atom inserted into the steroid skeleton at right.)
Sources[edit]
Saponins have historically been understood to be plant-derived, but they have also been isolated from marine organisms such as sea cucumber.[1][5] Saponins are indeed found in many plants,[1][6] and derive their name from the soapwort plant (genus Saponaria, family Caryophyllaceae), the root of which was used historically as a soap.[2] Saponins are also found in the botanical family Sapindaceae, with its defining genus Sapindus (soapberry or soapnut), and in the closely related families Aceraceae (maples) and Hippocastanaceae (horse chestnuts[citation needed]). It is also found heavily in Gynostemma pentaphyllum (Gynostemma, Cucurbitaceae) in a form called gypenosides, and ginseng or red ginseng (Panax, Araliaceae) in a form called ginsenosides. Saponins are also found in the unripe fruit of Manilkara zapota (also known as sapodillas), resulting in highly astringent properties. Within these families, this class of chemical compounds is found in various parts of the plant: leaves, stems, roots, bulbs, blossom and fruit.[7] Commercial formulations of plant-derived saponins, e.g., from the soap bark (or soapbark) tree, Quillaja saponaria, and those from other sources are available via controlled manufacturing processes, which make them of use as chemical and biomedical reagents.[8]
Test[edit]
- Froth Test
Uses plant Gogo (bark) Entada phaseoloides as control. The positive result shows a honeycomb froth that is higher than 2 cm that persists for 10 minutes or longer.
Blood Agar Media (BAM):
Is an agar cup semi-quantitative method that shows positive result of hemolytic halos.[9]
Role in plant ecology and impact on animal foraging[edit]
In plants, saponins may serve as anti-feedants,[2][4] and to protect the plant against microbes and fungi.[citation needed] Some plant saponins (e.g. from oat and spinach) may enhance nutrient absorption and aid in animal digestion. However, saponins are often bitter to taste, and so can reduce plant palatability (e.g., in livestock feeds), or even imbue them with life-threatening animal toxicity.[4] Some saponins are toxic to cold-blooded organisms and insects at particular concentrations.[4] Further research is needed to define the roles of these natural products in their host organisms, which have been described as "poorly understood" to date.[4]
Ethnobotany[edit]
Most saponins, which readily dissolve in water, are poisonous to fish.[10] Therefore, in ethnobotany, they are primarily known for their use by indigenous people in obtaining aquatic food sources.
Since prehistoric times, cultures throughout the world have used piscicidal (fish-killing) plants, mostly those containing saponins, for fishing.[11][12]
Although prohibited by law, fish poison plants are still widely used by indigenous tribes in Guyana.[13]
On the Indian Subcontinent, the Gond tribes are known for their use of plant extracts in poison fishing.[14]
Many of California's Native American tribes traditionally used soaproot, (genus Chlorogalum) and/or the root of various yucca species, which contain saponin, as a fish poison. They would pulverize the roots, mixing in water to create a foam, and then add the suds to a stream. This would kill or incapacitate the fish, which could be gathered easily from the surface of the water. Among the tribes using this technique were the Lassik, the Luiseño, and the Mattole.[15]
Research and uses[edit]
The amphipathic nature of saponins gives them activity as surfactants with potential ability to interact with cell membrane components, such as cholesterol and phospholipids, possibly making saponins useful for development of cosmetics and drugs.[16] Saponins have also been used as adjuvants in development of vaccines,[17] such as Quil A, an extract from the bark of Quillaja saponaria Molina (commonly called "quillaja").[16][18] This makes them of interest for possible use in subunit vaccines and vaccines directed against intracellular pathogens.[17] In their use as adjuvants in the production of vaccines, toxicity associated with sterol complexation remains a concern.[19]
While saponins are promoted commercially as dietary supplements and food ingredients,[20] and are used in traditional medicine preparations from licorice,[21][22] there is no high-quality clinical evidence that they have any beneficial effect on human health.[18] Quillaja is toxic when consumed in large amounts, involving possible liver damage, gastric pain, diarrhea, or other adverse effects.[18]
Saponins are used for their effects on ammonia emissions in animal feeding.[23]
See also[edit]
- Phytochemical
- Triterpenoid saponins
References[edit]
^ abcdefg Hostettmann, K.; A. Marston (1995). Saponins. Cambridge: Cambridge University Press. p. 3ff. ISBN 978-0-521-32970-5. OCLC 29670810..mw-parser-output cite.citationfont-style:inherit.mw-parser-output .citation qquotes:"""""""'""'".mw-parser-output .citation .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-ws-icon abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center.mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-maintdisplay:none;color:#33aa33;margin-left:0.3em.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ abcd "Saponins". Cornell University. 14 August 2008. Retrieved 23 February 2009.
^ "Project Summary: Functional Genomics of Triterpene Saponin Biosynthesis in Medicago Truncatula". Retrieved 23 February 2009.
^ abcde Foerster, Hartmut (22 May 2006). "MetaCyc Pathway: saponin biosynthesis I". Retrieved 23 February 2009.
^ Riguera, Ricardo (August 1997). "Isolating bioactive compounds from marine organisms". Journal of Marine Biotechnology. 5 (4): 187–193.
^ Liener, Irvin E (1980). Toxic constituents of plant foodstuffs. New York City: Academic Press. p. 161. ISBN 978-0-12-449960-7. OCLC 5447168.
[verification needed]
^ http://sun.ars-grin.gov:8080/npgspub/xsql/duke/plantdisp.xsql?taxon=691
^ "Saponin from quillaja bark". Sigma-Aldrich. Retrieved 23 February 2009.
^ Antibacterial activity of leave extracts of Nymphaea lotus (Nymphaeaceae) on Methicillin-resistant Staphylococcus aureus (MRSA) and Vancomycin resistant Staphylococcus aureus (VRSA) isolated from clinical samples. Akinjogunla OJ, Yah CS, Eghafona NO and Ogbemudia FO, Annals of Biological Research, 2010, 1 (2), pages 174–184
^ Howes, F. N. (1930), "Fish-poison plants", Bulletin of Miscellaneous Information (Royal Gardens, Kew), 1930 (4): 129–153, doi:10.2307/4107559, JSTOR 4107559
^ Jonathan G. Cannon, Robert A. Burton, Steven G. Wood, and Noel L. Owen (2004), "Naturally Occurring Fish Poisons from Plants", J. Chem. Educ., 81 (10): 1457, Bibcode:2004JChEd..81.1457C, doi:10.1021/ed081p1457CS1 maint: Uses authors parameter (link)
^ C. E. Bradley (1956), "Arrow and fish poison of the American southwest", Division of Biology, California Institute of Technology, 10 (4), pp. 362–366, doi:10.1007/BF02859766
^ Tinde Van Andel (2000), "The diverse uses of fish-poison plants in Northwest Guyana", Economic Botany, 54 (4): 500–512, doi:10.1007/BF02866548
^ Murthy E N, Pattanaik, Chiranjibi, Reddy, C Sudhakar, Raju, V S (March 2010), "Piscicidal plants used by Gond tribe of Kawal wildlife sanctuary, Andhra Pradesh, India", Indian Journal of Natural Products and Resources, 1 (1): 97–101CS1 maint: Multiple names: authors list (link)
^ Campbell, Paul (1999). Survival skills of native California. Gibbs Smith. p. 433. ISBN 978-0-87905-921-7.
^ ab Lorent, Joseph H.; Quetin-Leclercq, Joëlle; Mingeot-Leclercq, Marie-Paule (2014-11-28). "The amphiphilic nature of saponins and their effects on artificial and biological membranes and potential consequences for red blood and cancer cells". Organic and Biomolecular chemistry (Review). Royal Society of Chemistry. 12 (44): 8803–8822. doi:10.1039/c4ob01652a. ISSN 1477-0520. PMID 25295776.
^ ab Sun, Hong-Xiang; Xie, Yong; Ye, Yi-Ping (2009). "Advances in saponin-based adjuvants". Vaccine. 27 (12): 1787–1796. doi:10.1016/j.vaccine.2009.01.091. ISSN 0264-410X. PMID 19208455.
^ abc "Quillaja". Drugs.com. 2018. Retrieved 26 December 2018.
^ Skene, Caroline D.; Philip Sutton (1 September 2006). "Saponin-adjuvanted particulate vaccines for clinical use". Methods. 40 (1): 53–9. doi:10.1016/j.ymeth.2006.05.019. PMID 16997713.
^ "Tribulus". WebMD. Retrieved July 31, 2015.
^ Asl, Marjan Nassiri; Hossein Hosseinzadeh (1 June 2008). "Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds". Phytotherapy Research. 22 (6): 709–24. doi:10.1002/ptr.2362. PMID 18446848.
^ Xu R; Zhao W; Xu J; Shao B; Qin G (1996). Studies on bioactive saponins from Chinese medicinal plants. Advances in Experimental Medicine and Biology. 404. pp. 371–82. doi:10.1007/978-1-4899-1367-8_30. ISBN 978-1-4899-1369-2. PMID 8957308.
^ Zentner, Eduard (July 2011). "Effects of phytogenic feed additives containing quillaja saponaria on ammonia in fattening pigs" (PDF). Retrieved 27 November 2012.
External links[edit]
| Wikimedia Commons has media related to Saponins. |
- Medical Dictionary on Saponin
Saponins in Wine, by ScienceDaily, accessed Sep 9,2003- Molecular Expressions Phytochemical Gallery – Saponin
- Saponins: Suprising [sic] benefits of desert plants
Other uses of Quillaja Saponins and derived products, some works of different authors.- How to survive the world's worst diet
Quillia Extracts JECFA Food Additives Series 48
Categories:
- Saponins
(window.RLQ=window.RLQ||).push(function()mw.config.set("wgPageParseReport":"limitreport":"cputime":"0.812","walltime":"0.957","ppvisitednodes":"value":2951,"limit":1000000,"ppgeneratednodes":"value":0,"limit":1500000,"postexpandincludesize":"value":459097,"limit":2097152,"templateargumentsize":"value":1909,"limit":2097152,"expansiondepth":"value":16,"limit":40,"expensivefunctioncount":"value":9,"limit":500,"unstrip-depth":"value":1,"limit":20,"unstrip-size":"value":68884,"limit":5000000,"entityaccesscount":"value":6,"limit":400,"timingprofile":["100.00% 696.649 1 -total"," 49.21% 342.794 1 Template:Reflist"," 48.46% 337.605 28 Template:Navbox"," 19.63% 136.768 4 Template:Cite_book"," 14.63% 101.940 2 Template:Citation_needed"," 13.62% 94.879 1 Template:Phytochemical"," 12.37% 86.209 3 Template:Fix"," 8.43% 58.762 1 Template:Phenolic_compounds"," 8.13% 56.646 5 Template:Citation"," 7.51% 52.297 1 Template:Commons_category"],"scribunto":"limitreport-timeusage":"value":"0.379","limit":"10.000","limitreport-memusage":"value":6148739,"limit":52428800,"cachereport":"origin":"mw1323","timestamp":"20190330185726","ttl":2592000,"transientcontent":false););"@context":"https://schema.org","@type":"Article","name":"Saponin","url":"https://en.wikipedia.org/wiki/Saponin","sameAs":"http://www.wikidata.org/entity/Q207653","mainEntity":"http://www.wikidata.org/entity/Q207653","author":"@type":"Organization","name":"Contributors to Wikimedia projects","publisher":"@type":"Organization","name":"Wikimedia Foundation, Inc.","logo":"@type":"ImageObject","url":"https://www.wikimedia.org/static/images/wmf-hor-googpub.png","datePublished":"2004-04-12T08:08:30Z","dateModified":"2019-03-05T03:06:05Z","image":"https://upload.wikimedia.org/wikipedia/commons/5/58/Solanine_chemical_structure.png","headline":"class of chemical compounds"(window.RLQ=window.RLQ||).push(function()mw.config.set("wgBackendResponseTime":110,"wgHostname":"mw1325"););

