Concanavalin A
Concanavalin A
Jump to navigation
Jump to search
| Concanavalin A | |
|---|---|
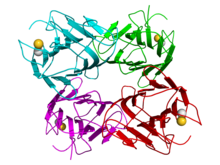 Crystallographic structure of a tetramer of jack bean concanavalin A (the monomers are colored cyan, green, red, and magenta respectively). Calcium (gold) and manganese cations (grey) are depicted as spheres.[1] | |
| Identifiers | |
| Organism | |
| Symbol | ConA |
| PDB | 3CNA More structures |
| UniProt | P81461 |
Concanavalin A (ConA) is a lectin (carbohydrate-binding protein) originally extracted from the jack-bean, Canavalia ensiformis. It is a member of the legume lectin family. It binds specifically to certain structures found in various sugars, glycoproteins, and glycolipids, mainly internal and nonreducing terminal α-D-mannosyl and α-D-glucosyl groups.[2][3] ConA is a plant mitogen, and is known for its ability to stimulate mouse T-cell subsets giving rise to four functionally distinct T cell populations, including precursors to suppressor T-cell;[4] one subset of human suppressor T-cells as well is sensitive to ConA.[4] ConA was the first lectin to be available on a commercial basis, and is widely used in biology and biochemistry to characterize glycoproteins and other sugar-containing entities on the surface of various cells.[5] It is also used to purify glycosylated macromolecules in lectin affinity chromatography,[6] as well as to study immune regulation by various immune cells.[4]
Contents
1 Structure and properties
2 Biological activity
3 References
4 External links
Structure and properties[edit]
Like most lectins, ConA is a homotetramer: each sub-unit (26.5KDa, 235 amino-acids, heavily glycated) binds a metallic atom (usually Mn2+ and a Ca2+). It has the D2 symmetry.[1] Its tertiary structure has been elucidated,[7] and the molecular basis of its interactions with metals as well as its affinity for the sugars mannose and glucose[8] are well known.
ConA binds specifically α-D-mannosyl and α-D-glucosyl residues (two hexoses differing only by the alcohol on carbon 2) in terminal position of ramified structures from B-Glycans (reach in α-mannose, or hybrid and bi-antennary glycanes complexes). It has 4 binding sites, corresponding to the 4 sub-units.[3] The molecular weight is 104-112KDa and the isoelectric point (pI) is in the range of 4.5-5.5.
Concanavalin A has a low-frequency wave number of 20 cm−1 in its Raman spectra.[9] This emission has been assigned to the breathing motion of the beta barrel consisting of 14 beta-strands in the concanavalin A molecule.[10]
ConA can also initiate cell division (mitogenesis) principally acting on T-lymphocytes, by stimulating the energy metabolism of thymocytes within seconds of exposure.[11]
Biological activity[edit]
Concanavalin A interacts with diverse receptors containing mannose carbohydrates, notably rhodopsin, blood group markers, insulin-receptor[12] the Immunoglobulins and the carcino-embryonary antigen (CEA). It also interacts with lipoproteins.[13]
ConA strongly agglutinates erythrocytes irrespective of blood-group, and various cancerous cells.[14][15][16] It was demonstrated that transformed cells and trypsin-treated normal cells do not agglutinate at 4 °C, thereby initiate suggesting that there is a temperature-sensitive step involved in ConA-mediated agglutination.[17][18]
ConA-mediated agglutination of other cell types has been reported, including muscle cells (myocytes),[19] B-lymphocytes (through surface Immunoglobulins),[20]fibroblasts,[21] rat thymocytes,[22] human fetal (but not adult) intestinal epithelial cells,[23] and adipocytes.[24]
ConA is a lymphocyte mitogen. Similar to phytohemagglutinin (PHA), it is a selective T cell mitogen relative to its effects on B cells. PHA and ConA bind and cross-link components of the T cell receptor, and their ability to activate T cells is dependent on expression of the T cell receptor.[25][26]
ConA interacts with the surface mannose residues of many microbes, like the bacteria E. coli,[27] and Bacillus subtilis[28] and the protist Dictyostelium discoideum.[29]
It has also been shown as a stimulator of several matrix metalloproteinases (MMPs).[30]
ConA has proven useful in applications requiring solid-phase immobilization of glycoenzymes, especially those that have proved difficult to immobilize by traditional covalent coupling. Using ConA-couple matrices, such enzymes may be immobilized in high quantities without a concurrent loss of activity and/or stability. Such noncovalent ConA-glycoenzyme couplings may be relatively easily reversed by competition with sugars or at acidic pH. If necessary for certain applications, these couplings can be converted to covalent bindings by chemical manipulation.[31]
A report from Taiwan (2009) demonstrated potent therapeutic effect of ConA against experimental hepatoma (liver cancer); in the study by Lei and Chang,[32] ConA was found to be sequestered more by hepatic tumor cells, in preference to surrounding normal hepatocytes. Internalization of ConA occurs preferentially to the mitochondria after binding to cell membrane glycoproteins, which triggers an autophagic cell death. ConA was found to partially inhibit tumor nodule growth independent of its lymphocyte activation; the eradication of the tumor in the murine in-situ hepatoma model in this study was additionally attributed to the mitogenic/lymphoproliferative action of ConA that may have activated a CD8+ T-cell-mediated, as well as NK- and NK-T cell-mediated, immune response in the liver.[32]
ConA intravitreal injection can be used in the modeling of proliferative vitreoretinopathy in rats.[33][34]
References[edit]
^ ab PDB: 3CNA; Hardman, Karl D.; Ainsworth, Clinton F. (1972). "Structure of concanavalin a at 2.4-Ang resolution". Biochemistry. 11 (26): 4910–9. doi:10.1021/bi00776a006. PMID 4638345..mw-parser-output cite.citationfont-style:inherit.mw-parser-output .citation qquotes:"""""""'""'".mw-parser-output .citation .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-ws-icon abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center.mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-maintdisplay:none;color:#33aa33;margin-left:0.3em.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ Goldstein, Irwin J.; Poretz, Ronald D. (2012). "Isolation, physicochemical characterization, and carbohydrate-binding specificity of lectins". In Liener, Irvin E.; Sharon, Nathan; Goldstein, Irwin J. The Lectins Properties, Functions and Applications in Biology and Medicine. Elsevier. pp. 33–247. ISBN 978-0-323-14444-5.
^ ab Sumner JB, Gralën N, Eriksson-Quensel IB (Apr 1938). "The Molecular Weights of Urease, Canavalin, Concanavalin a and Concanavalin B". Science. 87 (2261): 395–6. Bibcode:1938Sci....87..395S. doi:10.1126/science.87.2261.395. PMID 17746464.
^ abc Dwyer JM, Johnson C (Nov 1981). "The use of concanavalin A to study the immunoregulation of human T cells". Clinical and Experimental Immunology. 46 (2): 237–49. PMC 1536405. PMID 6461456.
^ Schiefer HG, Krauss H, Brunner H, Gerhardt U (Dec 1975). "Ultrastructural visualization of surface carbohydrate structures on mycoplasma membranes by concanavalin A". Journal of Bacteriology. 124 (3): 1598–600. PMC 236075. PMID 1104592.
^ GE Healthcare Life Sciences, Immobilized lectin[full citation needed]
^ Min W, Dunn AJ, Jones DH (Apr 1992). "Non-glycosylated recombinant pro-concanavalin A is active without polypeptide cleavage". The EMBO Journal. 11 (4): 1303–7. doi:10.1002/j.1460-2075.1992.tb05174.x. PMC 556578. PMID 1563347.
^ Loris R, Hamelryck T, Bouckaert J, Wyns L (Mar 1998). "Legume lectin structure". Biochimica et Biophysica Acta. 1383 (1): 9–36. doi:10.1016/S0167-4838(97)00182-9. PMID 9546043.
^ Painter PC, Mosher LE, Rhoads C (Jul 1982). "Low-frequency modes in the Raman spectra of proteins". Biopolymers. 21 (7): 1469–72. doi:10.1002/bip.360210715. PMID 7115900.
^ Chou KC (Aug 1985). "Low-frequency motions in protein molecules. Beta-sheet and beta-barrel". Biophysical Journal. 48 (2): 289–97. Bibcode:1985BpJ....48..289C. doi:10.1016/S0006-3495(85)83782-6. PMC 1329320. PMID 4052563.
^ Krauss S, Buttgereit F, Brand MD (Jun 1999). "Effects of the mitogen concanavalin A on pathways of thymocyte energy metabolism". Biochimica et Biophysica Acta. 1412 (2): 129–38. doi:10.1016/S0005-2728(99)00058-4. PMID 10393256.
^ Cuatrecasas P, Tell GP (Feb 1973). "Insulin-like activity of concanavalin A and wheat germ agglutinin--direct interactions with insulin receptors". Proceedings of the National Academy of Sciences of the United States of America. 70 (2): 485–9. Bibcode:1973PNAS...70..485C. doi:10.1073/pnas.70.2.485. JSTOR 62526. PMC 433288. PMID 4510292.
^ Harmony JA, Cordes EH (Nov 1975). "Interaction of human plasma low density lipoprotein with concanavalin A and with ricin". The Journal of Biological Chemistry. 250 (22): 8614–7. PMID 171260.
^ Betton GR (Nov 1976). "Agglutination reactions of spontaneous canine tumour cells, induced by concanavalin A, demonstrated by an isotopic assay". International Journal of Cancer. 18 (5): 687–96. doi:10.1002/ijc.2910180518. PMID 992901.
^ Kakizoe T, Komatsu H, Niijima T, Kawachi T, Sugimura T (Jun 1980). "Increased agglutinability of bladder cells by concanavalin A after administration of carcinogens". Cancer Research. 40 (6): 2006–9. PMID 7371036.
^ Becker FF, Shurgin A (Oct 1975). "Concanavalin A agglutination of cells from primary hepatocellular carcinomas and hepatic nodules induced by N-2-fluorenylacetamide". Cancer Research. 35 (10): 2879–83. PMID 168971.
^ Inbar M, Ben-Bassat H, Sachs L (Nov 1971). "A specific metabolic activity on the surface membrane in malignant cell-transformation". Proceedings of the National Academy of Sciences of the United States of America. 68 (11): 2748–51. Bibcode:1971PNAS...68.2748I. doi:10.1073/pnas.68.11.2748. JSTOR 61219. PMC 389516. PMID 4330939.
^ Sela BA, Lis H, Sharon N, Sachs L (Dec 1971). "Quantitation of N-acetyl-D-galactosamine-like sites on the surface membrane of normal and transformed mammalian cells". Biochimica et Biophysica Acta. 249 (2): 564–8. doi:10.1016/0005-2736(71)90132-5. PMID 4332414.
^ Gartner TK, Podleski TR (Dec 1975). "Evidence that a membrane bound lectin mediates fusion of L6 myoblasts". Biochemical and Biophysical Research Communications. 67 (3): 972–8. doi:10.1016/0006-291X(75)90770-6. PMID 1201086.
^ de Petris S (Apr 1975). "Concanavalin A receptors, immunoglobulins, and theta antigen of the lymphocyte surface. Interactions with concanavalin A and with Cytoplasmic structures". The Journal of Cell Biology. 65 (1): 123–46. doi:10.1083/jcb.65.1.123. PMC 2111157. PMID 1092699.
^ Noonan KD, Burger MM (Oct 1973). "The relationship of concanavalin A binding to lectin-initiated cell agglutination". The Journal of Cell Biology. 59 (1): 134–42. doi:10.1083/jcb.59.1.134. PMC 2110924. PMID 4201706.
^ Capo C, Garrouste F, Benoliel AM, Bongrand P, Ryter A, Bell GI (Aug 1982). "Concanavalin-A-mediated thymocyte agglutination: a model for a quantitative study of cell adhesion". Journal of Cell Science. 56: 21–48. PMID 7166565.
^ Weiser MM (Aug 1972). "Concanavalin A agglutination of intestinal cells from the human fetus". Science. 177 (4048): 525–6. Bibcode:1972Sci...177..525W. doi:10.1126/science.177.4048.525. PMID 5050484.
^ Cuatrecasas P (Mar 1973). "Interaction of wheat germ agglutinin and concanavalin A with isolated fat cells". Biochemistry. 12 (7): 1312–23. doi:10.1021/bi00731a011. PMID 4696755.
^ Weiss A, Shields R, Newton M, Manger B, Imboden J (Apr 1987). "Ligand-receptor interactions required for commitment to the activation of the interleukin 2 gene". Journal of Immunology. 138 (7): 2169–76. PMID 3104454.
^ Kanellopoulos JM, De Petris S, Leca G, Crumpton MJ (May 1985). "The mitogenic lectin from Phaseolus vulgaris does not recognize the T3 antigen of human T lymphocytes". European Journal of Immunology. 15 (5): 479–86. doi:10.1002/eji.1830150512. PMID 3873340.
^ Ofek I, Mirelman D, Sharon N (Feb 1977). "Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors". Nature. 265 (5595): 623–5. Bibcode:1977Natur.265..623O. doi:10.1038/265623a0. PMID 323718.
^ Doyle RJ, Birdsell DC (Feb 1972). "Interaction of concanavalin A with the cell wall of Bacillus subtilis". Journal of Bacteriology. 109 (2): 652–8. PMC 285189. PMID 4621684.
^ West CM, McMahon D (Jul 1977). "Identification of concanavalin A receptors and galactose-binding proteins in purified plasma membranes of Dictyostelium discoideum". The Journal of Cell Biology. 74 (1): 264–73. doi:10.1083/jcb.74.1.264. PMC 2109878. PMID 559679.
^ Yu M, Sato H, Seiki M, Thompson EW (Aug 1995). "Complex regulation of membrane-type matrix metalloproteinase expression and matrix metalloproteinase-2 activation by concanavalin A in MDA-MB-231 human breast cancer cells". Cancer Research. 55 (15): 3272–7. PMID 7614461.
^ Saleemuddin M, Husain Q (Apr 1991). "Concanavalin A: a useful ligand for glycoenzyme immobilization--a review". Enzyme and Microbial Technology. 13 (4): 290–5. doi:10.1016/0141-0229(91)90146-2. PMID 1367163.
^ ab Lei HY, Chang CP (2009). "Lectin of Concanavalin A as an anti-hepatoma therapeutic agent". Journal of Biomedical Science. 16: 10. doi:10.1186/1423-0127-16-10. PMC 2644972. PMID 19272170.
^ Erdiakov AK, Tikhonovich MV, Rzhavina EM, Gavrilova SA (May 2015). "[The characteristics of retina at the development of proliferative vitreoretinopathy in rats after intraocular injection of concanavalin a and dispase]". Rossiĭskii Fiziologicheskiĭ Zhurnal Imeni I.M. Sechenova / Rossiĭskaia Akademiia Nauk. 101 (5): 572–85. PMID 26263683.
^ Tikhonovich, Marina V.; Erdiakov, Aleksei K.; Gavrilova, Svetlana A. (2018). "Nonsteroid anti-inflammatory therapy suppresses the development of proliferative vitreoretinopathy more effectively than a steroid one". International Ophthalmology. 38 (4): 1365–1378. doi:10.1007/s10792-017-0594-3. ISSN 0165-5701. PMID 28639085.
External links[edit]
| Wikimedia Commons has media related to Canavalin. |
Concanavalin+A at the US National Library of Medicine Medical Subject Headings (MeSH)
Concanavalin+A+Receptors at the US National Library of Medicine Medical Subject Headings (MeSH)- Concanavalin A structure
- World of Lectin, Gateway to lectins
Proteopedia 1bxh con A in complex with methyl alpha1-2 mannobioside
Categories:
- Proteins
- Lectins
- Legume lectins
(window.RLQ=window.RLQ||).push(function()mw.config.set("wgPageParseReport":"limitreport":"cputime":"0.756","walltime":"0.887","ppvisitednodes":"value":2124,"limit":1000000,"ppgeneratednodes":"value":0,"limit":1500000,"postexpandincludesize":"value":97132,"limit":2097152,"templateargumentsize":"value":1629,"limit":2097152,"expansiondepth":"value":12,"limit":40,"expensivefunctioncount":"value":6,"limit":500,"unstrip-depth":"value":1,"limit":20,"unstrip-size":"value":113885,"limit":5000000,"entityaccesscount":"value":5,"limit":400,"timingprofile":["100.00% 791.047 1 -total"," 77.33% 611.726 1 Template:Reflist"," 60.93% 482.023 32 Template:Cite_journal"," 7.20% 56.973 1 Template:Infobox_nonhuman_protein"," 7.13% 56.425 1 Template:Full_citation_needed"," 6.34% 50.142 1 Template:Fix"," 6.26% 49.524 1 Template:Infobox"," 5.71% 45.143 1 Template:Commons_category"," 3.84% 30.394 1 Template:Category_handler"," 2.62% 20.756 1 Template:Lectins"],"scribunto":"limitreport-timeusage":"value":"0.507","limit":"10.000","limitreport-memusage":"value":5709701,"limit":52428800,"cachereport":"origin":"mw1241","timestamp":"20190330193932","ttl":2592000,"transientcontent":false);mw.config.set("wgBackendResponseTime":102,"wgHostname":"mw1242"););

