Prunasin
Prunasin
Jump to navigation
Jump to search
 | |
| Names | |
|---|---|
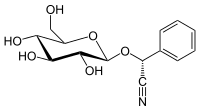
IUPAC name (2R)-2-Phenyl-2-[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyacetonitrile | |
| Other names (R)-Prunasin D-Prunasin D-Mandelonitrile-β-D-glucoside | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
ChEBI |
|
ChemSpider |
|
ECHA InfoCard | 100.002.489 |
EC Number | 202-738-0 |
KEGG |
|
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | C14H17NO6 |
Molar mass | 7002295291000000000♠295.291 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Infobox references | |
Prunasin is a cyanogenic glycoside related to amygdalin. Chemically, it is the glucoside of (R)-mandelonitrile.
Contents
1 Natural occurrences
2 Toxicity
3 Metabolism
4 References
Natural occurrences[edit]
Prunasin is found in species in the genus Prunus such as Prunus japonica or P. maximowiczii and in bitter almonds.[1] It is also found in leaves and stems of Olinia ventosa, O. radiata, O. emarginata and O. rochetiana[2] and in Acacia greggii.
It is also found in dandelion coffee, a coffee substitute.
Sambunigrin, a diastereomer of prunasin derived from (S)-mandelonitrile instead of it the (R)-isomer, has been isolated from leaves of the elder tree (Sambucus nigra)[3]
Toxicity[edit]
Prunasin is hydrolyzed to produce hydrogen cyanide. Plants containing prunasin may therefore be toxic to animals, particularly ruminants.[4]
Metabolism[edit]
Prunasin beta-glucosidase is an enzyme that uses (R)-prunasin and H2O to produce D-glucose and mandelonitrile.
Amygdalin beta-glucosidase is an enzyme that uses (R)-amygdalin and H2O to produce (R)-prunasin and D-glucose.
References[edit]
^ Sanchez-Perez, R.; Belmonte, F. S.; Borch, J.; Dicenta, F.; Møller, B. L.; Jørgensen, K. (2012). "Prunasin Hydrolases during Fruit Development in Sweet and Bitter Almonds". Plant Physiology. 158 (4): 1916–32. doi:10.1104/pp.111.192021. PMC 3320195. PMID 22353576..mw-parser-output cite.citationfont-style:inherit.mw-parser-output .citation qquotes:"""""""'""'".mw-parser-output .citation .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-ws-icon abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center.mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-maintdisplay:none;color:#33aa33;margin-left:0.3em.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ Nahrstedt, Adolf; Rockenbach, Jürgen (1993). "Occurrence of the cyanogenic glucoside prunasin and II corresponding mandelic acid amide glucoside in Olinia species (oliniaceae)". Phytochemistry. 34 (2): 433. doi:10.1016/0031-9422(93)80024-M.
^ Andrew Pengelly (2004), The Constituents of Medicinal Plants (2nd ed.), Allen & Unwin, pp. 44–45, ISBN 978-1-74114-052-1
^ Peter R. Cheeke (1989). Toxicants of Plant Origin: Glycosides. 2. CRC Press. p. 137.
Categories:
- Cyanogenic glycosides
- Alkaloids found in Fabaceae
- Plant toxins
- Glucosides
(window.RLQ=window.RLQ||).push(function()mw.config.set("wgPageParseReport":"limitreport":"cputime":"0.524","walltime":"0.670","ppvisitednodes":"value":5044,"limit":1000000,"ppgeneratednodes":"value":0,"limit":1500000,"postexpandincludesize":"value":57936,"limit":2097152,"templateargumentsize":"value":14691,"limit":2097152,"expansiondepth":"value":22,"limit":40,"expensivefunctioncount":"value":3,"limit":500,"unstrip-depth":"value":1,"limit":20,"unstrip-size":"value":12369,"limit":5000000,"entityaccesscount":"value":3,"limit":400,"timingprofile":["100.00% 635.033 1 -total"," 81.60% 518.169 1 Template:Chembox"," 44.08% 279.907 1 Template:Chembox_Identifiers"," 25.65% 162.906 3 Template:Chembox_headerbar"," 25.12% 159.546 9 Template:Trim"," 22.79% 144.730 1 Template:Chembox_Properties"," 17.88% 113.571 1 Template:Reflist"," 16.78% 106.533 1 Template:Chembox_Elements"," 15.99% 101.532 7 Template:Main_other"," 13.79% 87.590 1 Template:Chembox_parametercheck"],"scribunto":"limitreport-timeusage":"value":"0.240","limit":"10.000","limitreport-memusage":"value":5408599,"limit":52428800,"cachereport":"origin":"mw1239","timestamp":"20190329162412","ttl":2592000,"transientcontent":false);mw.config.set("wgBackendResponseTime":127,"wgHostname":"mw1329"););

