Artemisinin
Artemisinin
Jump to navigation
Jump to search
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ɑːrtɪˈmɪsɪnɪn/ |
| Synonyms | Artemisinine, qinghaosu |
| Routes of administration | Oral |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
PubChem CID |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
| ECHA InfoCard | 100.110.458 |
| Chemical and physical data | |
| Formula | C15H22O5 |
| Molar mass | 282.332 g/mol g·mol−1 |
| 3D model (JSmol) |
|
| Density | 1.24 ± 0.1 g/cm3 |
| Melting point | 152 to 157 °C (306 to 315 °F) |
| Boiling point | decomposes |
SMILES
| |
InChI
| |
.mw-parser-output .noboldfont-weight:normal (verify) | |
Artemisinin and its semisynthetic derivatives are a group of drugs used against malaria due to Plasmodium falciparum.[1] It was discovered in 1972 by Tu Youyou, a Chinese scientist, who was awarded half of the 2015 Nobel Prize in Medicine for her discovery.[2] Treatments containing an artemisinin derivative (artemisinin-combination therapies, ACTs) are now standard treatment worldwide for P. falciparum malaria. Artemisinin is isolated from the plant Artemisia annua, sweet wormwood, a herb employed in Chinese traditional medicine. A precursor compound can be produced using a genetically-engineered yeast, which is much more efficient than using the plant.[3]
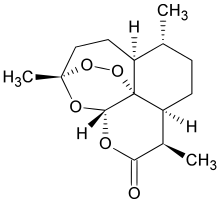
Chemically, artemisinin is a sesquiterpene lactone containing an unusual peroxide bridge. This endoperoxide 1,2,4-trioxane ring is responsible for the drug's mechanism of action. Few other natural compounds with such a peroxide bridge are known.[4]
Artemisinin and its derivatives have been used for the treatment of malarial and parastic worm (helminth) infections. They have the advantage over other drugs in having an ability to kill faster and kill all the life cycle stages of the parasites.[5] But low bioavailability, poor pharmacokinetic properties and high cost of the drugs are major drawbacks of their use.[6] Use of the drug by itself as a monotherapy is explicitly discouraged by the World Health Organization,[7] as there have been signs that malarial parasites are developing resistance to the drug. Therapies that combine artemisinin or its derivatives with some other antimalarial drug are the preferred treatment for malaria and are both effective and well tolerated in patients.[citation needed]
Contents
1 Medical use
1.1 Uncomplicated malaria
1.2 Severe malaria
1.3 Helminthiasis
1.4 Cancer
1.5 Autoimmune disease
2 Adverse effects
3 Chemistry
3.1 Derivatives
4 Mechanism of action
5 Resistance
6 Synthesis
6.1 Biosynthesis in A. annua
6.2 Chemical synthesis
6.3 Synthesis in engineered organisms
7 Production and price
8 Metabolism
9 History
9.1 Etymology
9.2 Discovery
10 References
11 External links
Medical use[edit]
Uncomplicated malaria[edit]
Artemisinins can be used alone, but this leads to a high rate of recrudescence (return of parasites) and other drugs are required to clear the body of all parasites and prevent recurrence. The World Health Organization (WHO) is pressuring manufacturers to stop making the uncompounded drug available to the medical community at large, aware of the catastrophe that would result if the malaria parasite developed resistance to artemisinins.[8]
The WHO has recommended artemisinin combination therapies (ACT) be the first-line therapy for P. falciparum malaria worldwide.[9] As short-acting drugs, artemisinin compounds are given with one or two long-acting drugs like amodiaquine, mefloquine, sulfadoxine/pyrimethamine or lumefantrine.[5] Combinations are effective because the artemisinin component kills the majority of parasites at the start of the treatment, while the more slowly eliminated partner drug clears the remaining parasites.[10]
Several fixed-dose ACTs are now available containing an artemisinin component and a partner drug which has a long half-life, such as mefloquine (ASMQ),[11]lumefantrine (Coartem), amodiaquine (ASAQ), piperaquine (Duo-Cotecxin), and pyronaridine (Pyramax). Increasingly, these combinations are being made to GMP standard. A separate issue concerns the quality of some artemisinin-containing products being sold in Africa and Southeast Asia.[12][13]
Artemisinins are not used for malaria prevention because of the extremely short activity (half-life) of the drug. To be effective, it would have to be administered multiple times each day.
Severe malaria[edit]
Artesunate administered by intravenous or intramuscular injection has proven superior to quinine in large, randomised controlled trials in both adults[14] and children.[15] Combining all trials comparing these two drugs, artesunate is associated with a mortality rate that is approximately 30% lower than that of quinine.[15] Reasons for this difference include reduced incidence of hypoglycaemia, easier administration and more rapid action against circulating and sequestered parasites. Artesunate is now recommended by the WHO for treatment of all cases of severe malaria. Effective treatment with ACT (artemisinin combination therapy) has proven to lower the morbidity and mortality from malaria within two years by around 70%.[16]
Helminthiasis[edit]
A serendipitous discovery was made in China in the early 1980s while searching for novel anthelmintics for schistosomiasis that artemisinin was effective against schistosomes,[17][18][19] the human blood flukes, which are the second-most prevalent parasitic infections, after malaria. Artemisinin and its derivatives are all potent anthelmintics.[20] Artemisinins were later found to possess a broad spectrum of activity against a wide range of trematodes, including Schistosoma japonicum, S. mansoni, S. haematobium, Clonorchis sinensis, Fasciola hepatica, and Opisthorchis viverrini. Clinical trials were also successfully conducted in Africa among patients with schistosomiasis.[21]
Cancer[edit]
Artemisinin and derivatives have been studied in many animal models. They can inhibit various molecules including catenin, adenosine monophosphate (AMP), cyclins and cyclin-dependent kinases that are involved in cancer development. They can cause cell death (apoptosis) in different cancer cells through activation of caspase-3 and caspase-9.[22] Their anticancer activity relies on their ability to produce reactive oxygen species. As of 2018, human clinical trials and other clinical research had been conducted in lung, colorectal, breast, liver, pancreas, glioma and cervical cancers, but the studies were too small to generalize from.[23]
Autoimmune disease[edit]
Artemisinin derivatives are known for their ability to suppress immune reactions such as inflammation. One derivative, SM934, was approved in 2015 by the China Food and Drug Administration for clinical trial as a drug for systemic lupus erythematosus (SLE).[24] Experiments in animal models have given good results. It can regulate T cell subsets, inhibit the activation of B cells, block the production of inflammatory cytokines and NF-κB signal transduction pathway.[25]
Adverse effects[edit]
Artemisinins are generally well tolerated at the doses used to treat malaria.[26] The side effects from the artemisinin class of medications are similar to the symptoms of malaria: nausea, vomiting, anorexia, and dizziness. Mild blood abnormalities have also been noted. A rare but serious adverse effect is allergic reaction.[26][27] One case of significant liver inflammation has been reported in association with prolonged use of a relatively high-dose of artemisinin for an unclear reason (the patient did not have malaria).[28] The drugs used in combination therapies can contribute to the adverse effects experienced by those undergoing treatment. Adverse effects in patients with acute P. falciparum malaria treated with artemisinin derivatives tend to be higher.[29]
Chemistry[edit]
Artemisinin belongs to a class of sesquiterpene lactones. It has a basic molecular weight of 282.147 Da. As a solid crystalline compound, it is poorly soluble in oils and water. Therefore, it is mostly applied through the digestive tract, either by oral or rectal administration. Only with chemical modifications that it has been prepared for injection. Artesunate is the only artemisinin compound available for all types of administration procedure.[30] An unusual component is an endoperoxide 1,2,4-trioxane ring as its functional group. This is the main antimalarial centre of the molecule. Modifications at carbon 10 (C10) position give rise to a variety of derivatives which are more powerful than the original compound.[31]
Derivatives[edit]
Because the physical properties of artemisinin itself, such as poor bioavailability, limit its effectiveness, semisynthetic derivatives of artemisinin have been developed. Derivatives of dihydroartemisinin were made since 1976. Artersunate, arteether and artemether were synthesised in 1986. Many derivatives have been produced of which artelinic acid, artemotil, artemisone, SM735, SM905, SM933, SM934, and SM1044 are among the most powerful compounds.[32][33]
There are also simplified analogs in preclinical research.[34]
A synthetic compound with a similar trioxolane structure (a ring containing three oxygen atoms) named arterolane[35] showed promise in in vitro testing. Phase II testing in patients with malaria was not as successful as hoped, but the manufacturer decided to start Phase III testing anyway.[36]
Mechanism of action[edit]
As of 2018, the exact mechanism of action of artemisinins was not known because of the complex chemical interactions involved.[37] Artemisinins do not directly attack malarial parasites or cells. They have to undergo chemical changes in the blood. Their functional group endoperoxide ring has to be activated first. Activation is done by cleavage of the endoperoxide ring. As the drug molecules come in contact with the haem (inside the haemoglobin of the red blood cells), the iron(II) oxide breaks the endoperoxide ring.[38] This process produces free radicals that in turn damage susceptible proteins, resulting in the death of the parasite.[39][40] In 2016 artemisinin was shown to bind to a large number of targets suggesting that it acts in a promiscuous manner.[41] Unlike other antimalarials which are active only on a particular stage of malarial parasite, artemisinin is able to kill all the life cycle stages.[42]
Resistance[edit]
Clinical evidence for artemisinin drug resistance in southeast Asia was first reported in 2008,[43] and was subsequently confirmed by a detailed study from western Cambodia.[44][45] Resistance in neighbouring Thailand was reported in 2012,[46] and in northern Cambodia, Vietnam and eastern Myanmar in 2014.[47][48] Emerging resistance was reported in southern Laos, central Myanmar and northeastern Cambodia in 2014.[47][48] The parasite's kelch gene on chromosome 13 appears to be a reliable molecular marker for clinical resistance in Southeast Asia.[49]
In April 2011, the WHO stated that resistance to the most effective antimalarial drug, artemisinin, could unravel national Indian malaria control programs, which have achieved significant progress in the last decade. WHO advocates the rational use of antimalarial drugs and acknowledges the crucial role of community health workers in reducing malaria in the region.[50]
Two main mechanisms of resistance drive Plasmodium resistance to antimalarial drugs. The first one is an efflux of the drug away from its action site due to mutations in different transporter genes (like pfcrt in chloroquine resistance) or an increased number of the gene copies (like pfmdr1 copy number in mefloquine resistance). The second is a change in the parasite target due to mutations in corresponding genes (like, at the cytosol level, dhfr and dhps in sulfadoxine-pyrimethamine resistance or, at the mitochondrion level, cytochrome b in atovaquone resistance). Resistance of Plasmodium falciparum to the new artemisinin compounds involves a novel mechanism corresponding to a quiescence phenomenon.[51]
Synthesis[edit]
Biosynthesis in A. annua[edit]
The biosynthesis of artemisinin is believed to involve the mevalonate pathway (MVA) and the cyclization of farnesyl diphosphate (FDP). It is not clear whether the non-mevalonate pathway can also contribute 5-carbon precursors (IPP or DMAPP), as occurs in other sesquiterpene biosynthetic systems. The routes from artemisinic alcohol to artemisinin remain controversial, and they differ mainly in when the reduction step takes place. Both routes suggested dihydroartemisinic acid as the final precursor to artemisinin. Dihydroartemisinic acid then undergoes photo-oxidation to produce dihydroartemisinic acid hydroperoxide. Ring expansion by the cleavage of hydroperoxide and a second oxygen-mediated hydroperoxidation finish the biosynthesis of artemisinin.

Chemical synthesis[edit]
The total synthesis of artemisinin has been performed from available organic starting materials, using basic organic reagents, many times. The first two total syntheses were a "remarkable... stereoselective synthesis" by Schmid and Hofheinz at Hoffmann-La Roche in Basel starting from (−)-isopulegol (13 steps, ~5% overall yield) and a concurrent synthesis by Zhou and coworkers at the Shanghai Institute of Organic Chemistry from (R)-(+)-citronellal (20 steps, ~0.3% overall yield).[52] Key steps of the Schmid–Hofheinz approach included an initial Ohrloff stereoselective hydroboration/oxidation to establish the "off-ring" methyl stereocenter on the propene side chain; two sequential lithium-reagent mediated alkylations that introduced all needed carbon atoms and that were, together highly diastereoselective; and further reduction, oxidation, and desilylation steps performed on this mono-carbocyclic intermediate, including a final singlet oxygen-utilizing photooxygenation and ene reaction, which, after acidic workup closed the three remaining oxacyclic rings of the desired product, artemisinin, in a single step.[52][53][54](In essence, the final oxidative ring closing operation in these syntheses accomplishes the closing three biosynthetic steps shown above.)
A wide variety of further routes continue to be explored, from early days until today, including total synthesis routes from (R)-(+)-pulegone, isomenthene,[52] and even 2-cyclohexen-1-one,[55] as well as routes better described as partial or semisyntheses from a more plentiful biosynthetic precursor, artemisinic acid—in the latter case, including some very short and very high yielding biomimetic synthesis examples (of Roth and Acton, and Haynes et al., 3 steps, 30% yield), which again feature the singlet oxygen ene chemistry.[56][52][57][58]
Synthesis in engineered organisms[edit]
The partnership to develop semisynthetic artemisinin was led by PATH’s Drug Development program (through an affiliation with OneWorld Health), with funding from the Bill & Melinda Gates Foundation. The project began in 2004, and initial project partners included the University of California, Berkeley (which provided the technology on which the project was based – a process that genetically altered yeast to produce artemisinic acid)[59] and Amyris (a biotechnology firm in California, which refined the process to enable large-scale production and developed scalable processes for transfer to an industrial partner).
In 2006, a team from UC Berkeley reported they had engineered Saccharomyces cerevisiae yeast to produce small amount of the precursor artemisinic acid. The synthesized artemisinic acid can then be transported out, purified and chemically converted into artemisinin that they claim will cost roughly US$0.25 per dose. In this effort of synthetic biology, a modified mevalonate pathway was used, and the yeast cells were engineered to express the enzyme amorphadiene synthase and a cytochrome P450 monooxygenase (CYP71AV1), both from A. annua. A three-step oxidation of amorpha-4,11-diene gives the resulting artemisinic acid.[60]
The Berkeley method was augmented using technology from various other organizations. The final successful technology is based on inventions licensed from UC Berkeley and the National Research Council (NRC) Plant Biotechnology Institute of Canada.
Commercial production of semisynthetic artemisinin is now underway at Sanofi's site in Garessio, Italy. This second source of artemisinin is poised to enable a more stable flow of key antimalarial treatments to those who need them most.[61] The production goal is set at 35 tonnes for 2013. It is expected to increase to 50–60 tons per year in 2014, supplying approximately one third of the global annual need for artemisinin.
On May 8, 2013, WHO's Prequalification of Medicines Programme announced the acceptability of semisynthetic artemisinin for use in the manufacture of active pharmaceutical ingredients submitted to WHO for prequalification, or that have already been qualified by WHO.[62] Sanofi’s active pharmaceutical ingredient (API) produced from semisynthetic artemisinin (artesunate) was also prequalified by WHO on May 8, 2013, making it the first semisynthetic artemisinin derivative prequalified.
In 2010, a team from Wageningen University reported they had engineered a close relative of tobacco, Nicotiana benthamiana, that can also produce the precursor artemisinic acid.[63]
Production and price[edit]
China and Vietnam provide 70% and East Africa 20% of the raw plant material.[citation needed] Seedlings are grown in nurseries and then transplanted into fields. It takes about 8 months for them to reach full size. The plants are harvested, the leaves are dried and sent to facilities where the artemisinin is extracted using a solvent, typically hexane. Alternative extraction methods have been proposed.[64] The market price for artemisinin has fluctuated widely, between US$120 and $1,200 per kilogram from 2005 to 2008.[65]
The Chinese company Artepharm created a combination artemisinin and piperaquine drug marketed as Artequick. In addition to clinical studies performed in China and southeast Asia, Artequick was used in large scale malaria eradication efforts in the Comoros. Those efforts, conducted in 2007, 2012, and 2013–14, produced a 95–97% reduction in the number of malaria cases in the Comoros.[66]
After negotiation with the WHO, Novartis and Sanofi-Aventis provide ACT drugs at cost on a nonprofit basis; however, these drugs are still more expensive than other malaria treatments.[67] Artesunate injection for severe malaria treatment is made by the Guilin Pharmaceutical factory in China where production has received WHO prequalification.[68] High-yield varieties of Artemisia are being produced by the Centre for Novel Agricultural Products at the University of York using molecular breeding techniques.[65]
Using seed supplied by Action for Natural Medicine (ANAMED), the World Agroforestry Centre (ICRAF) has developed a hybrid, dubbed A3, which can grow to a height of 3 metres and produce 20 times more artemisinin than wild varieties. In northwestern Mozambique, ICRAF is working together with a medical organisation, Médecins sans frontières, ANAMED and the Ministry of Agriculture and Rural Development to train farmers on how to grow the shrub from cuttings, and to harvest and dry the leaves to make artemisia tea.
In April 2013, Sanofi announced the launch[61] of a production facility in Garessio, Italy, to manufacture the antiplasmodial drug on a large scale. The partnership to create a new pharmaceutical manufacturing process was led by PATH’s Drug Development program (through an affiliation with OneWorld Health), with funding from the Bill & Melinda Gates Foundation and based on a modified biosynthetic process for artemisinic acid, initially designed by Jay Keasling at the University of California, Berkeley and optimized by Amyris. The reaction is followed by a photochemical process creating singlet oxygen to obtain the end product. Sanofi expects to produce 25 tons of artemisinin in 2013, ramping up the production to 55–60 tonnes in 2014. The price per kilogram will be US$350–400, roughly the same as the botanical source.[69] Despite concerns that this equivalent source would lead to the demise of companies, which produce this substance conventionally through extraction of A. annua biomass, an increased supply of this drug will likely produce lower prices and therefore increase the availability for ACTs treatment. In August 2014, Sanofi announced the release of the first batch of semisynthetic artemisinin. 1.7 million doses of Sanofi's artesunate amodiaquine Winthrop (ASAQ Winthrop), a fixed-dose artemisinin-based combination therapy will be shipped to half a dozen African countries over the next few months.[70]
A 2016 systematic review of four studies from East Africa concluded that subsidizing artemisinin-based combination (therapy ACT) in the private retail sector in combination with training and marketing led to increased availability of ACTs in stores, increased use of ACTs for febrile children under five years of age, and decrease in the use of older, less effective antimalarials among children under five years of age; the underlying studies did not determine if the children had malaria nor determine if there were health benefits.[71]
Metabolism[edit]
In the liver artemisinin is converted to different inactive metabolites such as deoxyartemisinin, deoxydihydroartemisinin, crystal 7, and 9,10-dihydrodeoxyartemisinin. The metabolites have lost the endoperoxide group and become ineffective. The reaction is catalysed by an enzyme CYP2B6, while another enzyme CYP3A4 acts as a secondary catalyst. In the absence of CYP2B6, CYP3A4 becomes the primary enzyme. These enzymes belong to cytochrome P450 group present in the smooth endoplasmic reticulum. Artemisinin derivatives are metabolised differently. They are first converted to dihydroartemisinin (DHA). DHA itself is a strong antimalarial molecule and is active in the blood circulation for two to three hours. The antimalarial activity of artesunate is actually only through DHA. (Artemisinin, arteether, artemether, etc. are directly antimalarials.) Artesunate is converted to DHA within a minute of its absorption. About 90% of the total DHA normally binds to blood plasma.[31] In the liver, cytochrome P450 enzyme system (including CYP2A6, CYP3A4, and CYP3A5) convert DHA into inactive metabolites. All the metabolites undergo glucuronidation after which they are excreted through the urine or faeces. UDP-glucuronosyltransferases, in particular UGT1A9 and UGT2B7, are responsible for the process. DHA is also removed through bile as minor glucuronides, such as tetrahydrofurano acetate. Due to fast metabolism, artemisinins are relatively safe drugs.[5]
History[edit]
Etymology[edit]
Artemisinin is an antimalarial lactone derived from qinghao (青蒿, Artemisia annua or sweet wormwood). The medicinal value of this plant has been known to the Chinese for at least 2,000 years. In 1596, Li Shizhen recommended tea made from qinghao specifically to treat malaria symptoms in his Compendium of Materia Medica. The genus name is derived from the Greek goddess Artemis and, more specifically, may have been named after Queen Artemisia II of Caria, a botanist and medical researcher in the fourth century BCE.[72]
Discovery[edit]
Artemisia annua is a common herb found in many parts of the world, and has been used by Chinese herbalists for more than 2000 years in the treatment of malaria. The earliest record dates back to 200 BCE, in the Fifty-two Prescriptions unearthed from the Mawangdui.[73] Its antimalarial application was first described in Zhouhou Beiji Fang (The Handbook of Prescriptions for Emergencies, Chinese: 肘后备急方), edited in the middle of the 4th century CE by Ge Hong; in that book, 43 malaria treatment methods were recorded.[74] Images of the original scientific papers that record the history of the discovery, have been available online since 2006.[75]

Artemisia annua
In 1967, a plant screening research program, under a secret military programme code-named "Project 523", was set up by the People's Liberation Army to find an adequate treatment for malaria; the program and early clinical work were ordered of Mao Zedong at the request of North Vietnamese leaders to provide assistance for their malaria-ridden army.[76] In the course of this research in 1972, Tu Youyou discovered artemisinin in the leaves of Artemisia annua.[77]
The drug is named qinghaosu in Chinese.[77] It was one of many candidates tested as possible treatments for malaria by Chinese scientists, from a list of nearly 5,000 traditional Chinese medicines.[citation needed]Tu Youyou also discovered that a low-temperature extraction process could be used to isolate an effective antimalarial substance from the plant. Tu says she was influenced by a traditional Chinese herbal medicine source The Handbook of Prescriptions for Emergency Treatments written in 340 CE by Ge Hong saying that this herb should be steeped in cold water.[78] This book contained the useful reference to the herb: "A handful of qinghao immersed with two litres of water, wring out the juice and drink it all."
Tu's team subsequently isolated a useful extract.[77] The extracted substance, once subject to purification, proved to be useful starting point to obtain purified artemisinin.[77] A 2012 review reported that artemisinin-based therapies were the most effective drugs for treatment of malaria at that time;[79] it was also reported to clear malaria parasites from patients' bodies faster than other drugs. In addition to artemisinin, Project 523 developed a number of products that can be used in combination with artemisinin, including lumefantrine, piperaquine, and pyronaridine.[77]
Results were published in the Chinese Medical Journal in 1979.[77][80] The research was met with skepticism at first, partly because the chemical structure of artemisinin, particularly the peroxide portion, appeared to be too unstable to be a viable drug.[4]
In the late 1990s, Novartis filed a new Chinese patent for a combination treatment with artemether and lumefantrine, providing the first artemisinin-based combination therapies (Coartem) at reduced prices to the World Health Organization.[81] In 2006, after artemisinin had become the treatment of choice for malaria, the WHO called for an immediate halt to single-drug artemisinin preparations in favor of combinations of artemisinin with another malaria drug, to reduce the risk of parasites developing resistance.[82]
In 2011, Tu Youyou was awarded the prestigious Lasker-DeBakey Clinical Medical Research Award for her role in the discovery and development of artemisinin.[77][83] On October 5, 2015, she was awarded half of the 2015 Nobel Prize in Physiology or Medicine for discovering artemisinin, "a drug that has significantly reduced the mortality rates for patients suffering from malaria".[2] The other half of the prize was awarded jointly to William C. Campbell and Satoshi Ōmura for discovering avermectin, "the derivatives of which have radically lowered the incidence of River blindness and Lymphatic filariasis, as well as showing efficacy against an expanding number of other parasitic diseases".[2]
References[edit]
This article contains public domain text from the CDC as cited
^ White, N. J. (July 1997). "Assessment of the pharmacodynamic properties of antimalarial drugs in vivo". Antimicrob. Agents Chemother. 41 (7): 1413–1422. doi:10.1128/AAC.41.7.1413. PMC 163932. PMID 9210658..mw-parser-output cite.citationfont-style:inherit.mw-parser-output .citation qquotes:"""""""'""'".mw-parser-output .citation .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-ws-icon abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center.mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-maintdisplay:none;color:#33aa33;margin-left:0.3em.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ abc "The Nobel Prize in Physiology or Medicine 2015" (PDF). Nobel Foundation. Retrieved 2015-10-07.
^ Arsenault, Patrick; Wobbe, Kristin; Weathers, Pamela (2008). "Recent Advances in Artemisinin Production Through Heterologous Expression". Current Medicinal Chemistry. 15 (27): 2886–2896. doi:10.2174/092986708786242813. PMC 2821817. PMID 18991643.
^ ab Brown, Geoff (July 2006). "Artemisinin and a new generation of antimalarial drugs". Education in Chemistry. Vol. 43 no. 4. Royal Society of Chemistry. pp. 97–99. Retrieved 2018-03-09.
^ abc Whirl-Carrillo, M; McDonagh, EM; Hebert, JM; Gong, L; Sangkuhl, K; Thorn, CF; Altman, RB; Klein, TE (2012). "Pharmacogenomics knowledge for personalized medicine". Clinical Pharmacology and Therapeutics. 92 (4): 414–417. doi:10.1038/clpt.2012.96. PMC 3660037. PMID 22992668.
^ "Development of Novel Antimalarials". MalariaWorld. September 6, 2010. Retrieved 2016-10-22.
^ "WHO calls for an immediate halt to provision of single-drug artemisinin malaria pills". WHO. 19 January 2006.
^ Rehwagen, C. (May 2006). "WHO ultimatum on artemisinin monotherapy is showing results". The BMJ. 332 (7551): 1176. doi:10.1136/bmj.332.7551.1176-b. PMC 1463909. PMID 16709988.
^ Guidelines for the Treatment of Malaria. Geneva: World Health Organization. 2006. ISBN 978-92-4-154694-2.
^ White, N. J. (April 2004). "Antimalarial drug resistance". Journal of Clinical Investigation. 113 (8): 1084–1092. doi:10.1172/JCI21682. PMC 385418. PMID 15085184.
^ Krudsood, S.; Looareesuwan, S.; Tangpukdee, N.; et al. (June 2010). "New Fixed-Dose Artesunate-Mefloquine Formulation against Multidrug-Resistant Plasmodium falciparum in Adults: a Comparative Phase IIb Safety and Pharmacokinetic Study with Standard-Dose Nonfixed Artesunate plus Mefloquine". Antimicrobial Agents and Chemotherapy. 54 (9): 3730–3737. doi:10.1128/AAC.01187-09. PMC 2935027. PMID 20547795.
^ "Malaria drugs recalled in Kenya". BBC News. 17 August 2007.
^ Newton, P.; Proux, S.; Green, M.; et al. (June 2001). "Fake artesunate in southeast Asia". Lancet. 357 (9272): 1948–1950. doi:10.1016/S0140-6736(00)05085-6. PMID 11425421.
^ Dondorp, A.; Nosten, F.; Stepniewska, K.; Day, N.; White, N. (2005). "Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial". Lancet. 366 (9487): 717–725. doi:10.1016/S0140-6736(05)67176-0. PMID 16125588.
^ ab Dondorp, A. M.; Fanello, C. I.; Hendriksen, I. C.; et al. (November 2010). "Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial". Lancet. 376 (9753): 1647–1657. doi:10.1016/S0140-6736(10)61924-1. PMC 3033534. PMID 21062666.
^ Bhattarai, Achuyt; Ali, Abdullah S.; Kachur, S. Patrick; Mårtensson, Andreas; Abbas, Ali K.; Khatib, Rashid; Al-Mafazy, Abdul-wahiyd; Ramsan, Mahdi; Rotllant, Guida (2007-11-06). "Impact of Artemisinin-Based Combination Therapy and Insecticide-Treated Nets on Malaria Burden in Zanzibar". PLoS Medicine. 4 (11): e309. doi:10.1371/journal.pmed.0040309. PMC 2062481. PMID 17988171.
^ Le, W.; You, J.; Mei, J.; Wang, G.; Xie, R. (1981). "Antischistosomal action of some qing hou su derivatives in infected mice". Acta Pharmaceutica Sinica. 8: 81–88.
^ Le, W.; You, J.; Yang, Y.; Mei, J.; Guo, H.; Yang, H.; Zhang, C. (1982). "Studies on the efficacy of artemether in experimental schistosomiasis". Acta Pharmaceutica Sinica. 17 (3): 187–193.
^ Le, W.; You, J.; Mei, J. (1983). "Chemotherapeutic effect of artesunate in experimental schistosomiasis". Acta Pharmaceutica Sinica. 18: 619–621.
^ Xiao, S. (2005). "Development of antischistosomal drugs in China, with particular consideration to praziquantel and the artemisinins". Acta Tropica. 96 (2–3): 153–167. doi:10.1016/j.actatropica.2005.07.010. PMID 16112072.
^ Keiser, J.; Utzinger, J. (2007). "Artemisinins and synthetic trioxolanes in the treatment of helminth infections". Current Opinion in Infectious Diseases. 20 (6): 605–612. doi:10.1097/QCO.0b013e3282f19ec4. PMID 17975411.
^ Konstat-Korzenny, Enrique; Ascencio-Aragón, Jorge; Niezen-Lugo, Sebastian; Vázquez-López, Rosalino (2018). "Artemisinin and Its Synthetic Derivatives as a Possible Therapy for Cancer". Medical Sciences. 6 (1): 19. doi:10.3390/medsci6010019. PMC 5872176. PMID 29495461.
^ Raffetin, A.; Bruneel, F.; Roussel, C.; Thellier, M.; Buffet, P.; Caumes, E.; Jauréguiberry, S. (February 5, 2018). "Use of artesunate in non-malarial indications" (PDF). Medecine et Maladies Infectieuses. 48 (4): 238–249. doi:10.1016/j.medmal.2018.01.004. PMID 29422423.
^ Shi, Chenchen; Li, Haipeng; Yang, Yifu; Hou, Lifei (2015). "Anti-Inflammatory and Immunoregulatory Functions of Artemisinin and Its Derivatives". Mediators of Inflammation. 2015: 435713. doi:10.1155/2015/435713. PMC 4415672. PMID 25960615.
^ Mu, Xiaozhen; Wang, Chenchen (2018). "Artemisinins—a Promising New Treatment for Systemic Lupus Erythematosus: a Descriptive Review". Current Rheumatology Reports. 20 (9): 55. doi:10.1007/s11926-018-0764-y. PMID 30056574.
^ ab Taylor, W. R.; White, N. J. (2004). "Antimalarial drug toxicity: a review". Drug Safety. 27 (1): 25–61. doi:10.2165/00002018-200427010-00003. PMID 14720085.
^ Leonardi, E.; Gilvary, G.; White, N. J.; Nosten, F. (2001). "Severe allergic reactions to oral artesunate: a report of two cases". Transactions of the Royal Society of Tropical Medicine and Hygiene. 95 (2): 182–183. doi:10.1016/S0035-9203(01)90157-9. PMID 11355556.
^ "Hepatitis Temporally Associated with an Herbal Supplement Containing Artemisinin — Washington, 2008". CDC.
^ Price, R.; et al. (1999). "Adverse effects in patients with acute falciparum malaria treated with artemisinin derivatives". American Journal of Tropical Medicine and Hygiene. 60 (4): 547–555. doi:10.4269/ajtmh.1999.60.547. PMID 10348227.
^ Morris, Carrie A; Duparc, Stephan; Borghini-Fuhrer, Isabelle; Jung, Donald; Shin, Chang-Sik; Fleckenstein, Lawrence (2011). "Review of the clinical pharmacokinetics of artesunate and its active metabolite dihydroartemisinin following intravenous, intramuscular, oral or rectal administration". Malaria Journal. 10 (1): 263. doi:10.1186/1475-2875-10-263. PMC 3180444. PMID 21914160.
^ ab Woodrow, C J; Haynes, R K; Krishna, S (2005). "Artemisinins". Postgraduate Medical Journal. 81 (952): 71–78. doi:10.1136/pgmj.2004.028399. PMC 1743191. PMID 15701735.
^ Li, Ying (2012). "Qinghaosu (artemisinin): Chemistry and pharmacology". Acta Pharmacologica Sinica. 33 (9): 1141–1146. doi:10.1038/aps.2012.104. PMC 4003104. PMID 22922345.
^ Aderibigbe, Blessing (2017). "Design of Drug Delivery Systems Containing Artemisinin and Its Derivatives". Molecules. 22 (2): 323. doi:10.3390/molecules22020323. PMC 6155641. PMID 28230749.
^ Posner, Gary H.; Parker, Michael H.; Northrop, John; Elias, Jeffrey S.; Ploypradith, Poonsakdi; Xie, Suji; Shapiro, Theresa A. (1999). "Orally Active, Hydrolytically Stable, Semisynthetic, Antimalarial Trioxanes in the Artemisinin Family". Journal of Medicinal Chemistry. 42 (2): 300–304. doi:10.1021/jm980529v. PMID 9925735.
^ Vennerstrom, J. L.; Arbe-Barnes, S.; Brun, R.; et al. (August 2004). "Identification of an antimalarial synthetic trioxolane drug development candidate". Nature. 430 (7002): 900–904. Bibcode:2004Natur.430..900V. doi:10.1038/nature02779. PMID 15318224.
^ Unnikrishnan, C. H. (September 21, 2007). "Blow to Ranbaxy drug research plans". livemint.com.
^ Krieger, Johannes; Smeilus, Toni; Kaiser, Marcel; Seo, Ean-Jeong; Efferth, Thomas; Giannis, Athanassios (2018). "Total Synthesis and Biological Investigation of (−)-Artemisinin: The Antimalarial Activity of Artemisinin Is not Stereospecific". Angewandte Chemie International Edition. 57 (27): 8293–8296. doi:10.1002/anie.201802015. PMID 29723442.
^ Tilley, Leann; Straimer, Judith; Gnädig, Nina F.; Ralph, Stuart A.; Fidock, David A. (2016). "Artemisinin Action and Resistance in Plasmodium falciparum". Trends in Parasitology. 32 (9): 682–696. doi:10.1016/j.pt.2016.05.010. PMC 5007624. PMID 27289273.
^ Winzeler, E. A.; M. J., Manary (2014). "Drug resistance genomics of the antimalarial drug artemisinin". Genome Biology. 15 (11): 544. doi:10.1186/s13059-014-0544-6. PMC 4283579. PMID 25470531.
^ Cravo, P.; Napolitano, H.; Culleton, R. (August 2015). "How genomics is contributing to the fight against artemisinin-resistant malaria parasites". Acta Tropica. 148: 1–7. doi:10.1016/j.actatropica.2015.04.007. PMID 25910626.
^ Wang, J.; Zhang, C.; Chia, W.; Loh, C.; Li, Z.; Lee, Y.; He, Y.; Yuan, L.; Lim, T.; Liu, M.; Liew, C.; Lee, Y.; Zhang, J.; Lu, N.; Lim, C.; Hua, Z.; Liu, B.; Shen, H.; Tan, K.; Lin, Q. (2015). "Haem-activated promiscuous targeting of artemisinin in Plasmodium falciparum". Nature Communications. 6: 10111. doi:10.1038/ncomms10111. PMC 4703832. PMID 26694030.
^ Aweeka, Francesca T; German, Polina I (2008). "Clinical Pharmacology of Artemisinin-Based Combination Therapies". Clinical Pharmacokinetics. 47 (2): 91–102. doi:10.2165/00003088-200847020-00002. PMID 18193915.
^ Noedl, H.; Se, Y.; Schaecher, K.; Smith, B. L.; Socheat, D.; Fukuda, M. M.; Artemisinin Resistance in Cambodia 1 (ARC1) Study Consortium (2008). "Evidence of artemisinin-resistant malaria in western Cambodia". New England Journal of Medicine. 359 (24): 2619–2620. doi:10.1056/NEJMc0805011. PMID 19064625.
^ Morelle, Rebecca (20 October 2015). "Drug-resistant malaria can infect African mosquitoes". BBC News. Retrieved 20 October 2015.
^ Dondorp, A. M.; Nosten, F. O.; Yi, P.; Das, D.; Phyo, A. P.; Tarning, J.; Lwin, K. M.; Ariey, F.; Hanpithakpong, W.; Lee, S. J.; Ringwald, P.; Silamut, K.; Imwong, M.; Chotivanich, K.; Lim, P.; Herdman, T.; An, S. S.; Yeung, S.; Singhasivanon, P.; Day, N. P. J.; Lindegardh, N.; Socheat, D.; White, N. J. (2009). "Artemisinin Resistance in Plasmodium falciparum Malaria". New England Journal of Medicine. 361 (5): 455–467. doi:10.1056/NEJMoa0808859. PMC 3495232. PMID 19641202.
^ Phyo, A. P.; Nkhoma, S.; Stepniewska, K.; et al. (2012). "Emergence of artemisinin-resistant malaria on the Western border of Thailand: a longitudinal study". Lancet. 379 (9830): 1960–1966. doi:10.1016/S0140-6736(12)60484-X. PMC 3525980. PMID 22484134.
^ ab Briggs, Helen (July 30, 2014). "Call for 'radical action' on drug-resistant malaria". BBC News. Retrieved 2013-07-30.
^ ab Ashley, Elizabeth A.; Dhorda, Mehul; et al. (July 31, 2014). "Spread of Artemisinin Resistance in Plasmodium falciparum Malaria". The New England Journal of Medicine. 371 (5): 411–423. doi:10.1056/NEJMoa1314981. PMC 4143591. PMID 25075834.
^ Ariey, F. D. R.; Witkowski, B.; Amaratunga, C.; Beghain, J.; Langlois, A. C.; Khim, N.; Kim, S.; Duru, V.; Bouchier, C.; Ma, L.; Lim, P.; Leang, R.; Duong, S.; Sreng, S.; Suon, S.; Chuor, C. M.; Bout, D. M.; Ménard, S.; Rogers, W. O.; Genton, B.; Fandeur, T.; Miotto, O.; Ringwald, P.; Le Bras, J.; Berry, A.; Barale, J. C.; Fairhurst, R. M.; Benoit-Vical, F. O.; Mercereau-Puijalon, O.; Ménard, D. (2013). "A molecular marker of artemisinin-resistant Plasmodium falciparum malaria". Nature. 505 (7481): 50–55. doi:10.1038/nature12876. PMC 5007947. PMID 24352242.
^ "Drugs immunity 'may' fail malaria fight". The Jakarta Post. April 23, 2011.
^ Ouji, Manel; Augereau, Jean-Michel; Paloque, Lucie; Benoit-Vical, Françoise (2018). "Plasmodium falciparum resistance to artemisinin-based combination therapies: A sword of Damocles in the path toward malaria elimination". Parasite. 25: 24. doi:10.1051/parasite/2018021. ISSN 1776-1042. PMC 5909375. PMID 29676250.
^ abcd Pirrung, Michael C.; Morehead, Andrew T., Jr (1997). "A Sesquidecade of Sesquiterpenes, 1980–1994: Part A. Acyclic and Monocyclic Sesquiterpenes, Part 1". In Goldsmith, D. The Total Synthesis of Natural Products. 10. New York: John Wiley & Sons. pp. 90–96. ISBN 978-0-470-12962-3.
^ Schmid, G.; Hofheinz, W. (1983). "Total Synthesis of qinghaosu". Journal of the American Chemical Society. 105 (3): 624–625. doi:10.1021/ja00341a054.
^ Acton, Nancy; Klayman, Daniel (October 1985). "Artemisitene, a New Sesquiterpene Lactone Endoperoxide fromArtemisia annua". Planta Medica. 51 (05): 441–442. doi:10.1055/s-2007-969543. ISSN 0032-0943.
^ Zhu, Chunyin; Cook, Silas P. (2012). "A Concise Synthesis of (+)-Artemisinin". Journal of the American Chemical Society. 134 (33): 13577–13579. doi:10.1021/ja3061479. PMID 22866604.
^ "Chemical constituents from Artemisia annua". China Journal of Chinese Materia Medica. 2014-12-15. doi:10.4268/cjcmm20142423. ISSN 1001-5302.
^ Lévesque, F.; Seeberger, P. H. (2012). "Continuous-Flow Synthesis of the Anti-Malaria Drug Artemisinin". Angewandte Chemie International Edition. 51 (7): 1706–1709. doi:10.1002/anie.201107446. PMID 22250044.
^ Turconi, Joël; Griolet, Frédéric; Guevel, Ronan; Oddon, Gilles; Villa, Roberto; Geatti, Andrea; Hvala, Massimo; Rossen, Kai; Göller, Rudolf; et al. (2014). "Semisynthetic artemisinin, the chemical path to industrial production". Organic Process Research & Development. 18 (3): 417–422. doi:10.1021/op4003196.
^ Ball, Philip (2016). "Man Made: A History of Synthetic Life". Distillations. 2 (1): 15–23. Retrieved 20 March 2018.
^ Ro, D. K.; Paradise, E. M.; Ouellet, M.; et al. (April 2006). "Production of the antimalarial drug precursor artemisinic acid in engineered yeast". Nature. 440 (7086): 940–943. Bibcode:2006Natur.440..940R. doi:10.1038/nature04640. PMID 16612385.
^ ab Pantjushenko, Elena. "Sanofi and PATH announce the launch of large-scale production of semisynthetic artemisinin against malaria". PATH.
^ Pantjushenko, Elena. "Semisynthetic artemisinin achieves WHO prequalification". PATH. Retrieved 8 February 2014.
^ van Herpen, T. W.; Cankar, K.; Nogueira, M.; Bosch, D.; Bouwmeester, H. J.; Beekwilder, J. (3 December 2010). Yang, Haibing, ed. "Nicotiana benthamiana as a Production Platform for Artemisinin Precursors". PLoS ONE. 5 (12): e14222. Bibcode:2010PLoSO...514222V. doi:10.1371/journal.pone.0014222. PMC 2997059. PMID 21151979.
^ Lapkin, Alexei A.; Peters, Martina; Greiner, Lasse; Chemat, Smain; Leonhard, Kai; Liauw, Marcel A.; Leitner, Walter (2010). "Screening of new solvents for artemisinin extraction process using ab initio methodology". Green Chemistry. 12 (2): 241–251. doi:10.1039/b922001a. and literature cited therein
^ ab "Report of the Artemisinin Enterprise Conference 2008" (PDF).
^ "Cure all?". The Economist. January 25, 2014. Retrieved 2016-10-22.
^ "Artemisinin combination therapies". CNAP Artemisia Project. University of York.
^ "Guilin Pharmaceutical ─ The world's first producer of WHO prequalified artesunate for injection for severe malaria". mmv.org. 2010.
^ Peplow, Mark (April 2013). "Sanofi launches malaria drug production to maintain stability in artemisinin availability". Chemistryworld Online. RSC. Retrieved 2013-04-19.
^ Palmer, Eric (19 August 2014). "Sanofi shipping new malaria treatment manufactured from 'semisynthetic artemisinin'". Fierce Pharma Manufacturing. Retrieved 2014-09-14.
^ Opiyo, Newton; Yamey, Gavin; Garner, Paul (9 March 2016). "Subsidising artemisinin-based combination therapy in the private retail sector". Cochrane Database of Systematic Reviews. 3: CD009926. doi:10.1002/14651858.cd009926.pub2. PMC 4916935. PMID 26954551.
^ Various (July 2014). "Etymologia: Artemisinin". Emerging Infectious Diseases. 20 (7): 1217. doi:10.3201/eid2007.ET2007. Retrieved July 4, 2014.
^ "Medical manuscripts from Ma Wang Dui". Qinghaosu Project.
^ "Zhou Hou Bei Ji Fang". Qinghaosu Project.
^ Burns, William. "Qinghaosu Project website". Blogspot. Retrieved 8 August 2014.
^ Jianfang, Zhang (2006). A Detailed Chronological Record of Project 523 and the Discovery and Development of Qinghaosu (Artemisinin). ISBN 9781622121649.
^ abcdefg Miller, L. H.; Su, X. (September 16, 2011). "Artemisinin: discovery from the Chinese herbal garden". Cell. 146 (6): 855–858. doi:10.1016/j.cell.2011.08.024. ISSN 0092-8674. PMC 3414217. PMID 21907397.
^ "Lasker Award Rekindles Debate Over Artemisinin's Discovery". Science. 2011-09-29. Retrieved 2014-01-07.
^ Fairhurst, R. M.; Nayyar, G. M.; Breman, J. G.; Hallett, R.; Vennerstrom, J. L.; Duong, S.; Ringwald, P.; Wellems, T. E.; Plowe, C. V.; Dondorp, A. M. (2012). "Artemisinin-resistant malaria: Research challenges, opportunities, and public health implications". The American Journal of Tropical Medicine and Hygiene. 87 (2): 231–241. doi:10.4269/ajtmh.2012.12-0025. PMC 3414557. PMID 22855752.
^ "Antimalaria studies on Qinghaosu". Chinese Medical Journal. 92 (12): 811–816. December 1979. PMID 117984.
^ Neil, D. M. (January 17, 2012). "For Intrigue, Malaria Drug Gets the Prize". New York Times. Retrieved 20 April 2013.
^ "WHO calls for an immediate halt to provision of single-drug artemisinin malaria pills" (Press release). World Health Organization. January 19, 2006.
^ Weise, Elizabeth (September 12, 2011). "'America's Nobel' awarded to Chinese scientist". USA Today. Retrieved 2011-09-12.
External links[edit]
Daviss, B. (2005). "Malaria, Science, and Social Responsibility: Nonprofit drug-development partnership seeks to cure the ills of developing nations". The Scientist. 19 (6): 42.- BBC Horizon documentary about artemisinin
Categories:
- Antimalarial agents
- Chinese discoveries
- Experimental cancer drugs
- Organic peroxides
- Sesquiterpene lactones
- Trioxanes
- Oxygen heterocycles
- Heterocyclic compounds (4 or more rings)
- ATPase inhibitors
- Traditional Chinese medicine
- Commercialization of traditional medicines
(window.RLQ=window.RLQ||).push(function()mw.config.set("wgPageParseReport":"limitreport":"cputime":"1.828","walltime":"2.115","ppvisitednodes":"value":9684,"limit":1000000,"ppgeneratednodes":"value":0,"limit":1500000,"postexpandincludesize":"value":362987,"limit":2097152,"templateargumentsize":"value":7831,"limit":2097152,"expansiondepth":"value":16,"limit":40,"expensivefunctioncount":"value":8,"limit":500,"unstrip-depth":"value":1,"limit":20,"unstrip-size":"value":281790,"limit":5000000,"entityaccesscount":"value":5,"limit":400,"timingprofile":["100.00% 1817.301 1 -total"," 46.14% 838.428 1 Template:Reflist"," 31.54% 573.204 1 Template:Drugbox"," 30.24% 549.522 56 Template:Cite_journal"," 24.53% 445.725 1 Template:Infobox"," 10.22% 185.641 1 Template:Lang"," 6.74% 122.459 11 Template:Navbox"," 5.53% 100.412 16 Template:Unbulleted_list"," 4.37% 79.381 14 Template:Cite_web"," 3.71% 67.433 3 Template:Citation_needed"],"scribunto":"limitreport-timeusage":"value":"1.046","limit":"10.000","limitreport-memusage":"value":27424148,"limit":52428800,"cachereport":"origin":"mw1256","timestamp":"20190408160206","ttl":2592000,"transientcontent":false);mw.config.set("wgBackendResponseTime":99,"wgHostname":"mw1264"););

