Lutein
Lutein
Jump to navigation
Jump to search
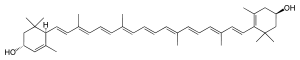 | |
 | |
| Names | |
|---|---|
IUPAC name β,ε-carotene-3,3'-diol | |
| Other names Luteine; trans-lutein; 4-[18-(4-Hydroxy-2,6,6-trimethyl-1-cyclohexenyl)-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaenyl]-3,5,5-trimethyl-cyclohex-2-en-1-ol Xanthophyll | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
ChEBI |
|
ChEMBL |
|
ChemSpider |
|
ECHA InfoCard | 100.004.401 |
E number | E161b (colours) |
PubChem CID |
|
UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | C40H56O2 |
Molar mass | 568.871 g/mol |
| Appearance | Red-orange crystalline solid |
Melting point | 190 °C (374 °F; 463 K)[1] |
Solubility in water | Insoluble |
Solubility in fats | Soluble |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Infobox references | |
Lutein (/ˈljuːtiɪn, -tiːn/;[2] from Latin luteus meaning "yellow") is a xanthophyll and one of 600 known naturally occurring carotenoids. Lutein is synthesized only by plants and like other xanthophylls is found in high quantities in green leafy vegetables such as spinach, kale and yellow carrots. In green plants, xanthophylls act to modulate light energy and serve as non-photochemical quenching agents to deal with triplet chlorophyll (an excited form of chlorophyll), which is overproduced at very high light levels, during photosynthesis. See xanthophyll cycle for this topic.
Lutein is obtained by animals by ingesting plants.[3] In the human retina, lutein is absorbed from blood specifically into the macula lutea,[4] although its precise role in the body is unknown.[3] Lutein is also found in egg yolks and animal fats.
Lutein is isomeric with zeaxanthin, differing only in the placement of one double bond. Lutein and zeaxanthin can be interconverted in the body through an intermediate called meso-zeaxanthin.[5] The principal natural stereoisomer of lutein is (3R,3′R,6′R)-beta,epsilon-carotene-3,3′-diol. Lutein is a lipophilic molecule and is generally insoluble in water. The presence of the long chromophore of conjugated double bonds (polyene chain) provides the distinctive light-absorbing properties. The polyene chain is susceptible to oxidative degradation by light or heat and is chemically unstable in acids.
Lutein is present in plants as fatty-acid esters, with one or two fatty acids bound to the two hydroxyl-groups. For this reason, saponification (de-esterfication) of lutein esters to yield free lutein may yield lutein in any ratio from 1:1 to 1:2 molar ratio with the saponifying fatty acid.
Contents
1 As a pigment
2 Role in human eyes
2.1 Macular degeneration
2.2 Cataract research
3 In diet
3.1 Safety
4 Commercial value
5 See also
6 References
7 External links
As a pigment[edit]
This xanthophyll, like its sister compound zeaxanthin, has primarily been used in food and supplement manufacturing as a colorant due to its yellow-red color.[3][6] Lutein absorbs blue light and therefore appears yellow at low concentrations and orange-red at high concentrations.
Many songbirds (like evening grosbeak, yellow warbler, common yellowthroat and Javan green magpie) deposit lutein obtained from the diet into growing tissues to color their feathers.[7][8]
Role in human eyes[edit]
Although lutein is concentrated in the macula – a small area of the retina responsible for three-color vision – the precise functional role of retinal lutein has not been determined.[3]
Macular degeneration[edit]
In 2013, findings of the Age-Related Eye Disease Study showed that a dietary supplement formulation containing lutein reduced progression of age-related macular degeneration (AMD) by 25 percent.[9][10] However, lutein and zeaxanthin had no overall effect on preventing AMD, but rather "the participants with low dietary intake of lutein and zeaxanthin at the start of the study, but who took an AREDS formulation with lutein and zeaxanthin during the study, were about 25 percent less likely to develop advanced AMD compared with participants with similar dietary intake who did not take lutein and zeaxanthin."[10]
In AREDS2, participants took one of four AREDS formulations: the original AREDS formulation, AREDS formulation with no beta-carotene, AREDS with low zinc, AREDS with no beta-carotene and low zinc. In addition, they took one of four additional supplement or combinations including lutein and zeaxanthin (10 mg and 2 mg), omega-3 fatty acids (1,000 mg), lutein/zeaxanthin and omega-3 fatty acids, or placebo. The study reported that there was no overall additional benefit from adding omega-3 fatty acids or lutein and zeaxanthin to the formulation. However, the study did find benefits in two subgroups of participants: those not given beta-carotene, and those who had little lutein and zeaxanthin in their diets. Removing beta-carotene did not curb the formulation's protective effect against developing advanced AMD, which was important given that high doses of beta-carotene had been linked to higher risk of lung cancers in smokers. It was recommended to replace beta-carotene with lutein and zeaxanthin in future formulations for these reasons.[9]
A more recent study investigated the effects of lutein and zeaxanthin on macular pigment and visual function in patients with early AMD. Daily intake of either 20 mg lutein alone or 10 mg lutein and 10 mg zeaxanthin was found to significantly increase the macular pigment content in comparison with the placebo group; the authors also reported a significant improvement in contrast sensitivity and a trend toward improvement in best-corrected visual acuity after 48 weeks of carotenoid supplementation. Furthermore, they found a significant correlation between these two parameters of visual function and the macular pigment content, suggesting that the increase in the latter underlies the improvement of visual function.[11]
Cataract research[edit]
There is preliminary epidemiological evidence that increasing lutein and zeaxanthin intake lowers the risk of cataract development.[3][12][13] Consumption of more than 2.4 mg of lutein/zeaxanthin daily from foods and supplements was significantly correlated with reduced incidence of nuclear lens opacities, as revealed from data collected during a 13- to 15-year period in one study.[14]
Two meta-analyses confirm a correlation between high diet content or high serum concentrations of lutein and zeaxanthin and a decrease in the risk of cataract.[15][16] There is only one published clinical intervention trial testing for an effect of lutein and zeaxanthin supplementation on cataracts. The AREDS2 trial enrolled subjects at risk for progression to advanced age-related macular degeneration. Overall, the group getting lutein (10 mg) and zeaxanthin (2 mg) were NOT less likely to progress to needing cataract surgery. The authors speculated that there may be a cataract prevention benefit for people with low dietary intake of lutein and zeaxanthin, but recommended more research.[17]
In diet[edit]
Lutein is a natural part of human diet when orange-yellow fruits and leafy green vegetables are consumed. According to the NHANES 2013-2014 survey, adults in the United States average 1.7 mg/day of lutein and zeaxanthin combined.[18] No recommended dietary allowance currently exists for lutein. Some positive health effects have been seen at dietary intake levels of 6–10 mg/day.[19] The only definitive side effect of excess lutein consumption is bronzing of the skin (carotenodermia).[citation needed]
As a food additive, lutein has the E number E161b (INS number 161b) and is extracted from the petals of African marigold (Tagetes erecta).[20] It is approved for use in the EU[21] and Australia and New Zealand[22] In the United States lutein may not be used as a food coloring for foods intended for human consumption, but can be added to animal feed. Example: lutein fed to chickens will show up in skin color and egg yolk color.[citation needed]
Some foods contain relatively high amounts of lutein:[3][12][23][24]
| Product | Lutein/zeaxanthin[3] (micrograms per 100 grams) |
|---|---|
nasturtium (yellow flowers, lutein levels only) | 45,000 [24] |
kale (raw) | 39,550 |
kale (cooked) | 18,246 |
dandelion leaves (raw) | 13,610 |
nasturtium (leaves, lutein levels only) | 13,600 [24] |
turnip greens (raw) | 12,825 |
spinach (raw) | 12,198 |
spinach (cooked) | 11,308 |
swiss chard (raw or cooked) | 11,000 |
turnip greens (cooked) | 8440 |
collard greens (cooked) | 7694 |
watercress (raw) | 5767 |
| garden peas (raw) | 2593 |
romaine lettuce | 2312 |
zucchini | 2125 |
brussels sprouts | 1590 |
pistachio nuts | 1205 |
broccoli | 1121 |
carrot (cooked) | 687 |
maize/corn | 642 |
egg (hard boiled) | 353 |
avocado (raw) | 271 |
carrot (raw) | 256 |
kiwifruit | 122 |
Safety[edit]
In humans, the Observed Safe Level (OSL) for lutein, based on a non-government organization evaluation, is 20 mg/day.[25] Although much higher levels have been tested without adverse effects and may also be safe, the data for intakes above the OSL are not sufficient for a confident conclusion of long-term safety.[3][25] Neither the U.S. Food and Drug Administration nor the European Food Safety Authority consider lutein an essential nutrient or have acted to set a tolerable upper intake level.[3]
Commercial value[edit]
The lutein market is segmented into pharmaceutical, dietary supplement, food, pet food, and animal and fish feed. The pharmaceutical market for lutein is estimated to be about US$190 million, and the nutraceutical and food categories are estimated to be about US$110 million. Pet food and other animal applications for lutein are estimated at US$175 million annually. This includes chickens (usually in combination with other carotenoids), to get color in egg yolks, and fish farms to color the flesh closer to wild-caught color. In the dietary supplement industry the major market for lutein is for products with claims of helping maintain eye health. Newer applications are emerging in oral and topical products for skin health. Skin health via orally consumed supplements is one of the fastest growing areas of the US$2 billion carotenoid market.[26]
See also[edit]
- Carotenoids
- List of phytochemicals in food
References[edit]
^ MSDS at Carl Roth (Lutein Rotichrom, German).
^ "Lutein", Random House Webster's Unabridged Dictionary.
^ abcdefghi "Carotenoids". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis. July 2016. Retrieved 10 August 2017..mw-parser-output cite.citationfont-style:inherit.mw-parser-output .citation qquotes:"""""""'""'".mw-parser-output .citation .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-ws-icon abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center.mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-maintdisplay:none;color:#33aa33;margin-left:0.3em.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ Bernstein, P. S.; Li, B; Vachali, P. P.; Gorusupudi, A; Shyam, R; Henriksen, B. S.; Nolan, J. M. (2015). "Lutein, Zeaxanthin, and meso-Zeaxanthin: The Basic and Clinical Science Underlying Carotenoid-based Nutritional Interventions against Ocular Disease". Progress in Retinal and Eye Research. 50: 34–66. doi:10.1016/j.preteyeres.2015.10.003. PMC 4698241. PMID 26541886.
^ Krinksy, Norman; Landrum, John; Bone, Richard (2003). "Biological Mechanisms of the Protective Role of Lutein and Zeaxanthin in the Eye". Annual Review of Nutrition. 23 (1): 171–201. doi:10.1146/annurev.nutr.23.011702.073307.
^ "Maintaining color stability". Natural Products Insider, Informa Exhibitions, LLC. 1 August 2006. Retrieved 10 August 2017.
^ McGraw KJ, Beebee MD, Hill GE, Parker RS (August 2003). "Lutein-based plumage coloration in songbirds is a consequence of selective pigment incorporation into feathers". Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology. 135 (4): 689–96. doi:10.1016/S1096-4959(03)00164-7. PMID 12892761.
^ Gill, Victoria. "Sold for a song: The forest birds captured for their tuneful voices". BBC News. Retrieved 31 December 2017.
^ ab "NIH study provides clarity on supplements for protection against blinding eye disease". US National Eye Institute, National Institutes of Health, Bethesda, MD. 5 May 2013. Retrieved 10 August 2017.
^ ab "The AREDS Formulation and Age-Related Macular Degeneration". US National Eye Institute, National Institutes of Health, Bethesda, MD. November 2011. Retrieved 10 August 2017.
^ Rothermel A, Kutuzov M (2015). "Benefits of Lutein and Zeaxanthin Intake in Patients with Age-Related Macular Degeneration, Retinitis Pigmentosa and Cataracts". Journal of Physiology and Pharmacology Advances. 5 (5): 634. doi:10.5455/jppa.20150509085929. ISSN 2251-7693.
[dead link]
^ ab SanGiovanni JP, Chew EY, Clemons TE, Ferris FL, Gensler G, Lindblad AS, Milton RC, Seddon JM, Sperduto RD (September 2007). "The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22". Archives of Ophthalmology (Chicago, Ill. : 1960). 125 (9): 1225–32. doi:10.1001/archopht.125.9.1225. PMID 17846363.
^ Moeller SM, Voland R, Tinker L, Blodi BA, Klein ML, Gehrs KM, Johnson EJ, Snodderly DM, Wallace RB, Chappell RJ, Parekh N, Ritenbaugh C, Mares JA (2008). "Associations between age-related nuclear cataract and lutein and zeaxanthin in the diet and serum in the Carotenoids in the Age-Related Eye Disease Study, an Ancillary Study of the Women's Health Initiative". Arch Ophthalmol. 126 (3): 354–64. doi:10.1001/archopht.126.3.354. PMC 2562026. PMID 18332316.
^ Barker Fm, 2nd (2010). "Dietary supplementation: effects on visual performance and occurrence of AMD and cataracts". Current Medical Research and Opinion. 26 (8): 2011–23. doi:10.1185/03007995.2010.494549. PMID 20590393.
^ Liu XH, Yu RB, Liu R, Hao ZX, Han CC, Zhu ZH, Ma L (2014). "Association between lutein and zeaxanthin status and the risk of cataract: a meta-analysis". Nutrients. 6 (1): 452–65. doi:10.3390/nu6010452. PMC 3916871. PMID 24451312.
^ Ma L, Hao ZX, Liu RR, Yu RB, Shi Q, Pan JP (2014). "A dose-response meta-analysis of dietary lutein and zeaxanthin intake in relation to risk of age-related cataract". Graefes Arch. Clin. Exp. Ophthalmol. 252 (1): 63–70. doi:10.1007/s00417-013-2492-3. PMID 24150707.
^ Chew EY, SanGiovanni JP, Ferris FL, Wong WT, Agron E, Clemons TE, Sperduto R, Danis R, Chandra SR, Blodi BA, Domalpally A, Elman MJ, Antoszyk AN, Ruby AJ, Orth D, Bressler SB, Fish GE, Hubbard GB, Klein ML, Friberg TR, Rosenfeld PJ, Toth CA, Bernstein P (2013). "Lutein/zeaxanthin for the treatment of age-related cataract: AREDS2 randomized trial report no. 4". JAMA Ophthalmol. 131 (7): 843–50. doi:10.1001/jamaophthalmol.2013.4412. PMID 23645227.
^ NHANES 2013-2014 survey results, reported as What We Eat In America
^ Seddon JM, Ajani UA, Sperduto RD (November 1994). "Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group". JAMA. 272 (18): 1413–20. doi:10.1001/jama.272.18.1413. PMID 7933422.
^ WHO/FAO Codex Alimentarius General Standard for Food Additives
^ UK Food Standards Agency: "Current EU approved additives and their E Numbers". Retrieved 2011-10-27.
^ Australia New Zealand Food Standards Code."Standard 1.2.4 - Labelling of ingredients". Retrieved 2011-10-27.
^ USDA National Nutrient Database for Standard Reference, Release 23 (2010) Archived 2015-03-03 at the Wayback Machine
^ abc Niizu, P.Y.; Delia B. Rodriguez-Amaya (2005). "Flowers and Leaves of Tropaeolum majus L. as Rich Sources of Lutein". Journal of Food Science. 70 (9): S605–S609. doi:10.1111/j.1365-2621.2005.tb08336.x. ISSN 1750-3841.
^ ab Shao A, Hathcock JN (2006). "Risk assessment for the carotenoids lutein and lycopene". Regulatory Toxicology and Pharmacology. 45 (3): 289–98. doi:10.1016/j.yrtph.2006.05.007. PMID 16814439. Retrieved 2016-07-17.The OSL risk assessment method indicates that the evidence of safety is strong at intakes up to 20mg/d for lutein, and 75 mg/d for lycopene, and these levels are identified as the respective OSLs. Although much higher levels have been tested without adverse effects and may be safe, the data for intakes above these levels are not sufficient for a confident conclusion of long-term safety.
^ FOD025C The Global Market for Carotenoids, BCC Research
External links[edit]
Categories:
- Carotenoids
- Food antioxidants
- Dietary antioxidants
- Food colorings
- Alcohols
- Cyclohexenes
- E-number additives
(window.RLQ=window.RLQ||).push(function()mw.config.set("wgPageParseReport":"limitreport":"cputime":"0.892","walltime":"1.137","ppvisitednodes":"value":6601,"limit":1000000,"ppgeneratednodes":"value":0,"limit":1500000,"postexpandincludesize":"value":132692,"limit":2097152,"templateargumentsize":"value":25470,"limit":2097152,"expansiondepth":"value":20,"limit":40,"expensivefunctioncount":"value":6,"limit":500,"unstrip-depth":"value":1,"limit":20,"unstrip-size":"value":71858,"limit":5000000,"entityaccesscount":"value":4,"limit":400,"timingprofile":["100.00% 1024.191 1 -total"," 58.58% 600.014 1 Template:Chembox"," 35.03% 358.749 1 Template:Chembox_Identifiers"," 28.64% 293.344 1 Template:Reflist"," 22.59% 231.360 3 Template:Chembox_headerbar"," 22.14% 226.754 9 Template:Trim"," 14.32% 146.616 7 Template:Main_other"," 12.80% 131.083 1 Template:Chembox_parametercheck"," 12.28% 125.803 13 Template:Cite_journal"," 11.55% 118.303 1 Template:Chembox_Properties"],"scribunto":"limitreport-timeusage":"value":"0.430","limit":"10.000","limitreport-memusage":"value":9115665,"limit":52428800,"cachereport":"origin":"mw1268","timestamp":"20190329134353","ttl":2592000,"transientcontent":false););"@context":"https://schema.org","@type":"Article","name":"Lutein","url":"https://en.wikipedia.org/wiki/Lutein","sameAs":"http://www.wikidata.org/entity/Q422067","mainEntity":"http://www.wikidata.org/entity/Q422067","author":"@type":"Organization","name":"Contributors to Wikimedia projects","publisher":"@type":"Organization","name":"Wikimedia Foundation, Inc.","logo":"@type":"ImageObject","url":"https://www.wikimedia.org/static/images/wmf-hor-googpub.png","datePublished":"2005-07-14T12:14:20Z","dateModified":"2019-02-18T20:09:09Z","image":"https://upload.wikimedia.org/wikipedia/commons/f/f9/Lutein_molecule_spacefill.png","headline":"chemical compound"(window.RLQ=window.RLQ||).push(function()mw.config.set("wgBackendResponseTime":1281,"wgHostname":"mw1268"););

