Estrogen
Estrogen
Jump to navigation
Jump to search
| Estrogen | |
|---|---|
| Drug class | |
 Estradiol, the major estrogen sex hormone in humans and a widely used medication. | |
| Class identifiers | |
| Use | Contraception, Menopause, hypogonadism, transgender women, prostate cancer, breast cancer, others |
| ATC code | G03C |
| Biological target | Estrogen receptors (ERα, ERβ, mERs (e.g., GPER, others)) |
| External links | |
| MeSH | D004967 |
| In Wikidata | |
Estrogen, or oestrogen, is the primary female sex hormone. It is responsible for the development and regulation of the female reproductive system and secondary sex characteristics. There are three major endogenous estrogens in females that have estrogenic hormonal activity: estrone, estradiol, and estriol. The estrane steroid estradiol is the most potent and prevalent of these.
Estrogens are synthesized in all vertebrates[1] as well as some insects.[2] Their presence in both vertebrates and insects suggests that estrogenic sex hormones have an ancient evolutionary history. The three major naturally occurring forms of estrogen in females are estrone (E1), estradiol (E2), and estriol (E3). Another type of estrogen called estetrol (E4) is produced only during pregnancy. Quantitatively, estrogens circulate at lower levels than androgens in both men and women.[3] While estrogen levels are significantly lower in males compared to females, estrogens nevertheless also have important physiological roles in males.[4]
Like all steroid hormones, estrogens readily diffuse across the cell membrane. Once inside the cell, they bind to and activate estrogen receptors (ERs) which in turn modulate the expression of many genes.[5] Additionally, estrogens bind to and activate rapid-signaling membrane estrogen receptors (mERs),[6][7] such as GPER (GPR30).[8]
In addition to their role as natural hormones, estrogens are used as medications, for instance in menopausal hormone therapy and hormonal birth control; for information on estrogens as medications, see the estrogen (medication) article.
.mw-parser-output .toclimit-2 .toclevel-1 ul,.mw-parser-output .toclimit-3 .toclevel-2 ul,.mw-parser-output .toclimit-4 .toclevel-3 ul,.mw-parser-output .toclimit-5 .toclevel-4 ul,.mw-parser-output .toclimit-6 .toclevel-5 ul,.mw-parser-output .toclimit-7 .toclevel-6 uldisplay:none
Contents
1 Types and examples
2 Biological function
2.1 Overview of actions
2.2 Female pubertal development
2.2.1 Breast development
2.3 Female reproductive system
2.4 Brain and behavior
2.4.1 Sex drive
2.4.2 Cognition
2.4.3 Mental health
2.4.4 Parenthood
2.4.5 Binge eating
2.4.6 Masculinization in rodents
2.5 Bone/skeletal system
2.6 Cardiovascular system
2.7 Immune system
2.8 Associated conditions
3 Biochemistry
3.1 Biosynthesis
3.2 Distribution
3.3 Metabolism
3.4 Excretion
3.5 Levels
4 Medical use
5 Chemistry
6 History
7 Society and culture
7.1 Etymology
7.2 Environment
7.3 Cosmetics
8 See also
9 References
10 External links
Types and examples[edit]

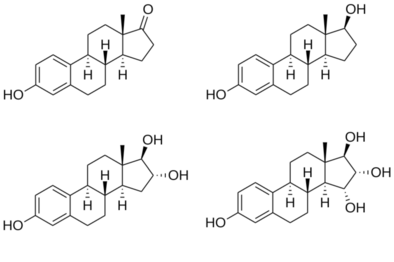
Chemical structures of major endogenous estrogens, including estrone (E1), estradiol (E2), estriol (E3), and estetrol (E4).[9]
The four major naturally occurring estrogens in women are estrone (E1), estradiol (E2), estriol (E3), and estetrol (E4). Estradiol is the predominant estrogen during reproductive years both in terms of absolute serum levels as well as in terms of estrogenic activity. During menopause, estrone is the predominant circulating estrogen and during pregnancy estriol is the predominant circulating estrogen in terms of serum levels. Given by subcutaneous injection in mice, estradiol is about 10-fold more potent than estrone and about 100-fold more potent than estriol.[10] Thus, estradiol is the most important estrogen in non-pregnant females who are between the menarche and menopause stages of life. However, during pregnancy this role shifts to estriol, and in postmenopausal women estrone becomes the primary form of estrogen in the body. Another type of estrogen called estetrol (E4) is produced only during pregnancy. All of the different forms of estrogen are synthesized from androgens, specifically testosterone and androstenedione, by the enzyme aromatase.
Minor endogenous estrogens, the biosyntheses of which do not involve aromatase, include 27-hydroxycholesterol, dehydroepiandrosterone (DHEA), 7-oxo-DHEA, 7α-hydroxy-DHEA, 16α-hydroxy-DHEA, 7β-hydroxyepiandrosterone, androstenedione (A4), androstenediol (A5), 3α-androstanediol, and 3β-androstanediol.[11][12] Some estrogen metabolites, such as the catechol estrogens 2-hydroxyestradiol, 2-hydroxyestrone, 4-hydroxyestradiol, and 4-hydroxyestrone, as well as 16α-hydroxyestrone, are also estrogens with varying degrees of activity.[13] The biological importance of these minor estrogens is not entirely clear.
Biological function[edit]

Reference ranges for the blood content of estradiol, the primary type of estrogen, during the menstrual cycle.[14]
The actions of estrogen are mediated by the estrogen receptor (ER), a dimeric nuclear protein that binds to DNA and controls gene expression. Like other steroid hormones, estrogen enters passively into the cell where it binds to and activates the estrogen receptor. The estrogen:ER complex binds to specific DNA sequences called a hormone response element to activate the transcription of target genes (in a study using an estrogen-dependent breast cancer cell line as model, 89 such genes were identified).[15] Since estrogen enters all cells, its actions are dependent on the presence of the ER in the cell. The ER is expressed in specific tissues including the ovary, uterus and breast. The metabolic effects of estrogen in postmenopausal women has been linked to the genetic polymorphism of the ER.[16]
While estrogens are present in both men and women, they are usually present at significantly higher levels in women of reproductive age. They promote the development of female secondary sexual characteristics, such as breasts, and are also involved in the thickening of the endometrium and other aspects of regulating the menstrual cycle. In males, estrogen regulates certain functions of the reproductive system important to the maturation of sperm[17][18][19] and may be necessary for a healthy libido.[20]
| Estrogen | ER RBA (%) |
|---|---|
| Estradiol | 100 |
| Estrone | 11 |
| Estriol | 10 |
| Estetrol | 0.5 |
| 2-Hydroxyestradiol | 23–24 |
| 4-Hydroxyestradiol | 43–45 |
| 2-Methoxyestradiol | 0.05 |
| 4-Methoxyestradiol | 1.3–13 |
| 2-Hydroxyestrone | 2–3 |
| 4-Hydroxyestrone | 10–11 |
| 2-Methoxyestrone | 0.01 |
| 4-Methoxyestrone | 0.1–0.13 |
| 17α-Estradiol | 32 |
| Estradiol valerate | 2 |
| Estrone sulfate | 2 |
| Equilin | 40 |
| Equilenin | 7 |
| 17β-Dihydroequilin | 47 |
| 17α-Dihydroequilin | 31 |
| 17β-Dihydroequilenin | 46 |
| 17α-Dihydroequilenin | 43 |
| Ethinylestradiol | 100 |
| Mestranol | 1 |
Notes: Rat proteins were used for the assays. Sources: See template. | |
| Estrogen | ER RBA (%) | Uterine weight (%) | ||
|---|---|---|---|---|
| Control | – | 100 | ||
| Estradiol | 100 | 506 | ||
| Estriol | 10 | 468 | ||
| 2-Hydroxyestradiol | 24 | 285 | ||
| 2-Methoxyestradiol | 0.05 | 101 | ||
| 4-Hydroxyestradiol | 45 | ? | ||
| 4-Methoxyestradiol | 13 | 260 | ||
| 2-Hydroxyestrone | 2 | 130 | ||
| 2-Methoxyestrone | 0.01 | ? | ||
| 4-Hydroxyestrone | 11 | 351 | ||
| 4-Methoxyestrone | 0.13 | 338 | ||
Notes: ER (rat uterine cytosol) RBA + estrogenicity (change in rat uterine wet weight) of estrogen metabolites. Sources: See template. | ||||
Overview of actions[edit]
- Structural
- Mediate formation of female secondary sex characteristics
- Accelerate metabolism
- Increase fat store
- Stimulate endometrial growth
- Increase uterine growth
- Increase vaginal lubrication
- Thicken the vaginal wall
- Maintenance of vessel and skin
- Reduce bone resorption, increase bone formation
- Mediate formation of female secondary sex characteristics
Protein synthesis- Increase hepatic production of binding proteins
- Increase hepatic production of binding proteins
Coagulation- Increase circulating level of factors 2, 7, 9, 10, plasminogen
- Decrease antithrombin III
- Increase platelet adhesiveness
- Increase vWF (estrogen -> Angiotensin II -> Vasopressin)
- Increase PAI-1 and PAI-2 also through Angiotensin II
- Increase circulating level of factors 2, 7, 9, 10, plasminogen
Lipid- Increase HDL, triglyceride
- Decrease LDL, fat deposition
- Increase HDL, triglyceride
- Fluid balance
- Salt (sodium) and water retention
- Increase cortisol, SHBG
Gastrointestinal tract- Reduce bowel motility
- Increase cholesterol in bile
Melanin- Increase pheomelanin, reduce eumelanin
- Increase pheomelanin, reduce eumelanin
- Cancer
- Support hormone-sensitive breast cancers (see section below)
Lung function- Promotes lung function by supporting alveoli (in rodents but probably in humans).[21]
Uterus lining- Estrogen together with progesterone promotes and maintains the uterus lining in preparation for implantation of fertilized egg and maintenance of uterus function during gestation period, also upregulates oxytocin receptor in myometrium
Ovulation- Surge in estrogen level induces the release of luteinizing hormone, which then triggers ovulation by releasing the egg from the Graafian follicle in the ovary.
Sexual behavior- Promotes sexual receptivity in estrus,[22] and induces lordosis behavior.[23] In non-human mammals, it also induces estrus (in heat) prior to ovulation, which also induces lordosis behavior. Female non-human mammals are not sexually receptive without the estrogen surge, i.e., they have no mating desire when not in estrus.
- Regulates the stereotypical sexual receptivity behavior; this lordosis behavior is estrogen-dependent, which is regulated by the ventromedial nucleus of the hypothalamus.[24]
Sex drive is dependent on androgen levels[25] only in the presence of estrogen, but without estrogen, free testosterone level actually decreases sexual desire (instead of increases sex drive), as demonstrated for those women who have hypoactive sexual desire disorder, and the sexual desire in these women can be restored by administration of estrogen (using oral contraceptive).[26] In non-human mammals, mating desire is triggered by estrogen surge in estrus.
Female pubertal development[edit]
Estrogens are responsible for the development of female secondary sexual characteristics during puberty, including breast development, widening of the hips, and female fat distribution. Conversely, androgens are responsible for pubic and body hair growth, as well as acne and axillary odor.
Breast development[edit]
Estrogen, in conjunction with growth hormone (GH) and its secretory product insulin-like growth factor 1 (IGF-1), is critical in mediating breast development during puberty, as well as breast maturation during pregnancy in preparation of lactation and breastfeeding.[27][28] Estrogen is primarily and directly responsible for inducing the ductal component of breast development,[29][30][31] as well as for causing fat deposition and connective tissue growth.[29][30] It is also indirectly involved in the lobuloalveolar component, by increasing progesterone receptor expression in the breasts[29][31][32] and by inducing the secretion of prolactin.[33][34] Allowed for by estrogen, progesterone and prolactin work together to complete lobuloalveolar development during pregnancy.[30][35]
Androgens such as testosterone powerfully oppose estrogen action in the breasts, such as by reducing estrogen receptor expression in them.[36][37]
Female reproductive system[edit]
Estrogens are responsible for maturation and maintenance of the vagina and uterus, and are also involved in ovarian function, such as maturation of ovarian follicles. In addition, estrogens play an important role in regulation of gonadotropin secretion. For these reasons, estrogens are required for female fertility.
Brain and behavior[edit]
Sex drive[edit]
Estrogens are involved in libido (sex drive) in both women and men.
Cognition[edit]
Verbal memory scores are frequently used as one measure of higher level cognition. These scores vary in direct proportion to estrogen levels throughout the menstrual cycle, pregnancy, and menopause. Furthermore, estrogens when administered shortly after natural or surgical menopause prevents decreases in verbal memory. In contrast, estrogens have little effect on verbal memory if first administered years after menopause.[38] Estrogens also have positive influences on other measures of cognitive function.[39] However the effect of estrogens on cognition is not uniformly favorable and is dependent on the timing of the dose and the type of cognitive skill being measured.[40]
The protective effects of estrogens on cognition may be mediated by estrogens anti-inflammatory effects in the brain.[41] Studies have also shown that the Met allele gene and level of estrogen mediates the efficiency of prefrontal cortex dependent working memory tasks.[42][43]
Mental health[edit]
Estrogen is considered to play a significant role in women's mental health. Sudden estrogen withdrawal, fluctuating estrogen, and periods of sustained low estrogen levels correlate with significant mood lowering. Clinical recovery from postpartum, perimenopause, and postmenopause depression has been shown to be effective after levels of estrogen were stabilized and/or restored.[44][45][46]Menstrual exacerbation (including menstrual psychosis) is typically triggered by low estrogen levels,[47] and is often mistaken for premenstrual dysphoric disorder.[48]
Compulsions in male lab mice, such as those in obsessive-compulsive disorder (OCD), may be caused by low estrogen levels. When estrogen levels were raised through the increased activity of the enzyme aromatase in male lab mice, OCD rituals were dramatically decreased. Hypothalamic protein levels in the gene COMT are enhanced by increasing estrogen levels which are believed to return mice that displayed OCD rituals to normal activity. Aromatase deficiency is ultimately suspected which is involved in the synthesis of estrogen in humans and has therapeutic implications in humans having obsessive-compulsive disorder.[49]
Local application of estrogen in the rat hippocampus has been shown to inhibit the re-uptake of serotonin. Contrarily, local application of estrogen has been shown to block the ability of fluvoxamine to slow serotonin clearance, suggesting that the same pathways which are involved in SSRI efficacy may also be affected by components of local estrogen signaling pathways.[50]
Parenthood[edit]
Studies have also found that fathers had lower levels of cortisol and testosterone but higher levels of estrogen (estradiol) compared to non-fathers.[51]
Binge eating[edit]
Estrogen may play a role in suppressing binge eating. Hormone replacement therapy using estrogen may be a possible treatment for binge eating behaviors in females. Estrogen replacement has been shown to suppress binge eating behaviors in female mice.[52] The mechanism by which estrogen replacement inhibits binge-like eating involves the replacement of serotonin (5-HT) neurons. Women exhibiting binge eating behaviors are found to have increased brain uptake of neuron 5-HT, and therefore less of the neurotransmitter serotonin in the cerebrospinal fluid.[53] Estrogen works to activate 5-HT neurons, leading to suppression of binge like eating behaviors.[52]
It is also suggested that there is an interaction between hormone levels and eating at different points in the female menstrual cycle. Research has predicted increased emotional eating during hormonal flux, which is characterized by high progesterone and estradiol levels that occur during the mid-luteal phase. It is hypothesized that these changes occur due to brain changes across the menstrual cycle that are likely a genomic effect of hormones. These effects produce menstrual cycle changes, which result in hormone release leading to behavioral changes, notably binge and emotional eating. These occur especially prominently among women who are genetically vulnerable to binge eating phenotypes.[54]
Binge eating is associated with decreased estradiol and increased progesterone.[55] Klump et al.[56] Progesterone may moderate the effects of low estradiol (such as during dysregulated eating behavior), but that this may only be true in women who have had clinically diagnosed binge episodes (BEs). Dysregulated eating is more strongly associated with such ovarian hormones in women with BEs than in women without BEs.[56]
The implantation of 17β-estradiol pellets in ovariectomized mice significantly reduced binge eating behaviors and injections of GLP-1 in ovariectomized mice decreased binge-eating behaviors.[52]
The associations between binge eating, menstrual-cycle phase and ovarian hormones correlated.[55][57][57][58]
Masculinization in rodents[edit]
In rodents, estrogens (which are locally aromatized from androgens in the brain) play an important role in psychosexual differentiation, for example, by masculinizing territorial behavior;[59] the same is not true in humans.[60] In humans, the masculinizing effects of prenatal androgens on behavior (and other tissues, with the possible exception of effects on bone) appear to act exclusively through the androgen receptor.[61] Consequently, the utility of rodent models for studying human psychosexual differentiation has been questioned.[62]
Bone/skeletal system[edit]
Estrogens are responsible for both the pubertal growth spurt, which causes an acceleration in linear growth, and epiphyseal closure, which limits height and limb length, in both females and males. In addition, estrogens are responsible for bone maturation and maintenance of bone mineral density throughout life. Due to hypoestrogenism, the risk of osteoporosis increases during menopause.
Cardiovascular system[edit]
Women suffer less from heart disease due to vasculo-protective action of estrogen which helps in preventing atherosclerosis.[63] It also helps in maintaining the delicate balance between fighting infections and protecting arteries from damage thus lowering the risk of cardiovascular disease.[64]
Immune system[edit]
Estrogen has anti-inflammatory properties and helps in mobilization of polymorphonuclear white blood cells or neutrophils.[64]
Associated conditions[edit]
Estrogens are implicated in various estrogen-dependent conditions, such as ER-positive breast cancer, as well as a number of genetic conditions involving estrogen signaling or metabolism, such as estrogen insensitivity syndrome, aromatase deficiency, and aromatase excess syndrome.
Biochemistry[edit]
Biosynthesis[edit]
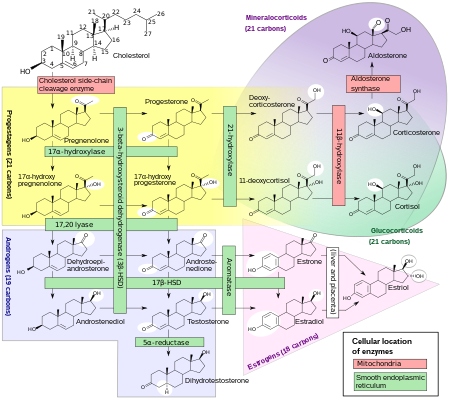
Steroidogenesis, showing estrogens at bottom right as in pink triangle.[65]
Estrogens, in females, are produced primarily by the ovaries, and during pregnancy, the placenta.[66]Follicle-stimulating hormone (FSH) stimulates the ovarian production of estrogens by the granulosa cells of the ovarian follicles and corpora lutea. Some estrogens are also produced in smaller amounts by other tissues such as the liver, pancreas, bone, adrenal glands, skin, brain, adipose tissue,[67] and the breasts.[68] These secondary sources of estrogens are especially important in postmenopausal women.[69]
The pathway of estrogen biosynthesis in extragonadal tissues is different. These tissues are not able to synthesize C19 steroids, and therefore depend on C19 supplies from other tissues[69] and the level of aromatase.[70]
In females, synthesis of estrogens starts in theca interna cells in the ovary, by the synthesis of androstenedione from cholesterol. Androstenedione is a substance of weak androgenic activity which serves predominantly as a precursor for more potent androgens such as testosterone as well as estrogen. This compound crosses the basal membrane into the surrounding granulosa cells, where it is converted either immediately into estrone, or into testosterone and then estradiol in an additional step. The conversion of androstenedione to testosterone is catalyzed by 17β-hydroxysteroid dehydrogenase (17β-HSD), whereas the conversion of androstenedione and testosterone into estrone and estradiol, respectively is catalyzed by aromatase, enzymes which are both expressed in granulosa cells. In contrast, granulosa cells lack 17α-hydroxylase and 17,20-lyase, whereas theca cells express these enzymes and 17β-HSD but lack aromatase. Hence, both granulosa and theca cells are essential for the production of estrogen in the ovaries.
Estrogen levels vary through the menstrual cycle, with levels highest near the end of the follicular phase just before ovulation.
Note that in males, estrogen is also produced by the Sertoli cells when FSH binds to their FSH receptors.
Distribution[edit]
Estrogens are plasma protein bound to albumin and/or sex hormone-binding globulin in the circulation.
Metabolism[edit]
Estrogens are metabolized via hydroxylation by cytochrome P450 enzymes such as CYP1A1 and CYP3A4 and via conjugation by estrogen sulfotransferases (sulfation) and UDP-glucuronyltransferases (glucuronidation). In addition, estradiol is dehydrogenated by 17β-hydroxysteroid dehydrogenase into the much less potent estrogen estrone. These reactions occur primarily in the liver, but also in other tissues.
Estrogen metabolism in humans[71][72][73] |
Excretion[edit]
Estrogens are excreted primarily by the kidneys as conjugates via the urine.
Levels[edit]
| Group | E2 (prod) | E2 (levels) | E1 (levels) | Ratio |
|---|---|---|---|---|
Pubertal girlsa Tanner stage I (childhood) Tanner stage II (ages 8–12) Tanner stage III (ages 10–13) Tanner stage IV (ages 11–14) Tanner stage V (ages 12–15) Follicular (days 1–14) Luteal (days 15–28) | ? ? ? ? ? ? | 9 (<9–20) pg/mL 15 (<9–30) pg/mL 27 (<9–60) pg/mL 55 (16–85) pg/mL 50 (30–100) pg/mL 130 (70–300) pg/mL | 13 (<9–23) pg/mL 18 (10–37) pg/mL 26 (17–58) pg/mL 36 (23–69) pg/mL 44 (30–89) pg/mL 75 (39–160) pg/mL | ? ? ? ? ? ? |
Prepubertal boys | ? | 2–8 pg/mL | ? | ? |
Premenopausal women Early follicular phase (days 1–4) Mid follicular phase (days 5–9) Late follicular phase (days 10–14) Luteal phase (days 15–28) Oral contraceptive (anovulatory) | 30–100 µg/day 100–160 µg/day 320–640 µg/day 300 µg/day ? | 40–60 pg/mL 60–100 pg/mL 200–400 pg/mL 190 pg/mL 12–50 pg/mL | 40–60 pg/mL ? 170–200 pg/mL 100–150 pg/mL ? | 0.5–1 ? 1–2 1.5 ? |
Postmenopausal women | 18 µg/day | 5–20 pg/mL | 30–70 pg/mL | 0.3–0.8 |
Pregnant women First trimester (weeks 1–12) Second trimester (weeks 13–26) Third trimester (weeks 27–40) | ? ? ? | 1,000–5,000 pg/mL 5,000–15,000 pg/mL 10,000–40,000 pg/mL | ? ? ? | ? ? ? |
| Mena | 20–60 µg/day | 27 (20–55) pg/mL | 20–90 pg/mL | 0.4–0.6 |
Footnotes: a = Format is "Mean value (range)". Sources: See template. | ||||
Medical use[edit]
Estrogens are used as medications, mainly in hormonal contraception and hormone replacement therapy.[74]
Chemistry[edit]
Structures of major endogenous estrogens |
The estrogen steroid hormones are estrane steroids.
History[edit]
In 1929, Adolf Butenandt and Edward Adelbert Doisy independently isolated and purified estrone, the first estrogen to be discovered.[75] Then, estriol and estradiol were discovered in 1930 and 1933, respectively. Shortly following their discovery, estrogens, both natural and synthetic, were introduced for medical use. Examples include estriol glucuronide (Emmenin, Progynon), estradiol benzoate, conjugated estrogens (Premarin), diethylstilbestrol, and ethinylestradiol.
The word estrogen derives from Ancient Greek. It is derived from "oestros[76]" (a periodic state of sexual activity in female mammals), and genos(generating).[76] It was first published in the early 1920s and referenced as "oestrin".[77] With the years, American English adapted the spelling of estrogen to fit with its phonetic pronunciation. Nevertheless, both estrogen and oestrogen are used nowadays, yet some still wish to maintain its original spelling as it reflects the origin of the word.
Society and culture[edit]
Etymology[edit]
The name estrogen is derived from the Greek οἶστρος (oistros), literally meaning "verve or inspiration" but figuratively sexual passion or desire,[78] and the suffix -gen, meaning "producer of".
Environment[edit]
A range of synthetic and natural substances that possess estrogenic activity have been identified in the environment and are referred to xenoestrogens.[79]
- Synthetic substances such as bisphenol A as well as metalloestrogens (e.g., cadmium).
- Plant products with estrogenic activity are called phytoestrogens (e.g., coumestrol, daidzein, genistein, miroestrol).
- Those produced by fungi are known as mycoestrogens (e.g., zearalenone).
Estrogens are among the wide range of endocrine-disrupting compounds (EDCs) because they have high estrogenic potency. When an EDC makes its way into the environment, it may cause male reproductive dysfunction to wildlife.[80] The estrogen excreted from farm animals makes its way into fresh water systems.[81] During the germination period of reproduction the fish are exposed to low levels of estrogen which may cause reproductive dysfunction to male fish.[82][83]
Cosmetics[edit]
Some hair shampoos on the market include estrogens and placental extracts; others contain phytoestrogens. In 1998, there were case reports of four prepubescent African-American girls developing breasts after exposure to these shampoos.[84] In 1993, the FDA determined that not all over-the-counter topically applied hormone-containing drug products for human use are generally recognized as safe and effective and are misbranded. An accompanying proposed rule deals with cosmetics, concluding that any use of natural estrogens in a cosmetic product makes the product an unapproved new drug and that any cosmetic using the term "hormone" in the text of its labeling or in its ingredient statement makes an implied drug claim, subjecting such a product to regulatory action.[85]
In addition to being considered misbranded drugs, products claiming to contain placental extract may also be deemed to be misbranded cosmetics if the extract has been prepared from placentas from which the hormones and other biologically active substances have been removed and the extracted substance consists principally of protein. The FDA recommends that this substance be identified by a name other than "placental extract" and describing its composition more accurately because consumers associate the name "placental extract" with a therapeutic use of some biological activity.[85]
See also[edit]
- List of steroid abbreviations
References[edit]
^ Ryan KJ (August 1982). "Biochemistry of aromatase: significance to female reproductive physiology". Cancer Research. 42 (8 Suppl): 3342s–3344s. PMID 7083198..mw-parser-output cite.citationfont-style:inherit.mw-parser-output .citation qquotes:"""""""'""'".mw-parser-output .citation .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-ws-icon abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center.mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-maintdisplay:none;color:#33aa33;margin-left:0.3em.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ Mechoulam R, Brueggemeier RW, Denlinger DL (September 2005). "Estrogens in insects" (PDF). Cellular and Molecular Life Sciences. 40 (9): 942–944. doi:10.1007/BF01946450.
^ Burger HG (April 2002). "Androgen production in women". Fertility and Sterility. 77 Suppl 4: S3–5. doi:10.1016/S0015-0282(02)02985-0. PMID 12007895.
^ Lombardi G, Zarrilli S, Colao A, Paesano L, Di Somma C, Rossi F, De Rosa M (June 2001). "Estrogens and health in males". Molecular and Cellular Endocrinology. 178 (1–2): 51–5. doi:10.1016/S0303-7207(01)00420-8. PMID 11403894.
^ Whitehead SA, Nussey S (2001). Endocrinology: an integrated approach. Oxford: BIOS: Taylor & Francis. ISBN 978-1-85996-252-7.
^ Soltysik K, Czekaj P (April 2013). "Membrane estrogen receptors – is it an alternative way of estrogen action?". Journal of Physiology and Pharmacology. 64 (2): 129–42. PMID 23756388.
^ Micevych PE, Kelly MJ (2012). "Membrane estrogen receptor regulation of hypothalamic function". Neuroendocrinology. 96 (2): 103–10. doi:10.1159/000338400. PMC 3496782. PMID 22538318.
^ Prossnitz ER, Arterburn JB, Sklar LA (February 2007). "GPR30: A G protein-coupled receptor for estrogen". Molecular and Cellular Endocrinology. 265–266: 138–42. doi:10.1016/j.mce.2006.12.010. PMC 1847610. PMID 17222505.
^ Coelingh Bennink HJ, Holinka CF, Diczfalusy E (2008). "Estetrol review: profile and potential clinical applications". Climacteric. 11 Suppl 1: 47–58. doi:10.1080/13697130802073425. PMID 18464023.
^ A. Labhart (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 548–. ISBN 978-3-642-96158-8.
^ Baker ME (March 2013). "What are the physiological estrogens?". Steroids. 78 (3): 337–40. doi:10.1016/j.steroids.2012.12.011. PMID 23313336.
^ Miller KK, Al-Rayyan N, Ivanova MM, Mattingly KA, Ripp SL, Klinge CM, Prough RA (January 2013). "DHEA metabolites activate estrogen receptors alpha and beta". Steroids. 78 (1): 15–25. doi:10.1016/j.steroids.2012.10.002. PMC 3529809. PMID 23123738.
^ Bhavnani BR, Nisker JA, Martin J, Aletebi F, Watson L, Milne JK (2000). "Comparison of pharmacokinetics of a conjugated equine estrogen preparation (premarin) and a synthetic mixture of estrogens (C.E.S.) in postmenopausal women". Journal of the Society for Gynecologic Investigation. 7 (3): 175–83. doi:10.1016/s1071-5576(00)00049-6. PMID 10865186.
^ Häggström, Mikael (2014). "Reference ranges for estradiol, progesterone, luteinizing hormone and follicle-stimulating hormone during the menstrual cycle". WikiJournal of Medicine. 1 (1). doi:10.15347/wjm/2014.001. ISSN 2002-4436.
^ Lin CY, Ström A, Vega VB, Kong SL, Yeo AL, Thomsen JS, Chan WC, Doray B, Bangarusamy DK, Ramasamy A, Vergara LA, Tang S, Chong A, Bajic VB, Miller LD, Gustafsson JA, Liu ET (2004). "Discovery of estrogen receptor alpha target genes and response elements in breast tumor cells". Genome Biology. 5 (9): R66. doi:10.1186/gb-2004-5-9-r66. PMC 522873. PMID 15345050.
^ Darabi M, Ani M, Panjehpour M, Rabbani M, Movahedian A, Zarean E (2011). "Effect of estrogen receptor β A1730G polymorphism on ABCA1 gene expression response to postmenopausal hormone replacement therapy". Genetic Testing and Molecular Biomarkers. 15 (1–2): 11–5. doi:10.1089/gtmb.2010.0106. PMID 21117950.
^ Raloff J (6 December 1997). "Science News Online (12/6/97): Estrogen's Emerging Manly Alter Ego". Science News. Retrieved 4 March 2008.
^ Hess RA, Bunick D, Lee KH, Bahr J, Taylor JA, Korach KS, Lubahn DB (December 1997). "A role for oestrogens in the male reproductive system". Nature. 390 (6659): 509–12. doi:10.1038/37352. PMC 5719867. PMID 9393999.
^ "Estrogen Linked To Sperm Count, Male Fertility". Science Blog. Retrieved 4 March 2008.
^ Hill RA, Pompolo S, Jones ME, Simpson ER, Boon WC (December 2004). "Estrogen deficiency leads to apoptosis in dopaminergic neurons in the medial preoptic area and arcuate nucleus of male mice". Molecular and Cellular Neurosciences. 27 (4): 466–76. doi:10.1016/j.mcn.2004.04.012. PMID 15555924.
^ Massaro D, Massaro GD (December 2004). "Estrogen regulates pulmonary alveolar formation, loss, and regeneration in mice". American Journal of Physiology. Lung Cellular and Molecular Physiology. 287 (6): L1154–9. doi:10.1152/ajplung.00228.2004. PMID 15298854.
^ Christensen A, Dewing P, Micevych P (November 2011). "Membrane-initiated estradiol signaling induces spinogenesis required for female sexual receptivity". The Journal of Neuroscience. 31 (48): 17583–9. doi:10.1523/JNEUROSCI.3030-11.2011. PMC 4709636. PMID 22131419.
^ Handa RJ, Ogawa S, Wang JM, Herbison AE (January 2012). "Roles for oestrogen receptor β in adult brain function". Journal of Neuroendocrinology. 24 (1): 160–73. doi:10.1111/j.1365-2826.2011.02206.x. PMC 3348521. PMID 21851428.
^ Kow LM, Pfaff DW (May 1998). "Mapping of neural and signal transduction pathways for lordosis in the search for estrogen actions on the central nervous system". Behavioural Brain Research. 92 (2): 169–80. doi:10.1016/S0166-4328(97)00189-7. PMID 9638959.
^ Warnock JK, Swanson SG, Borel RW, Zipfel LM, Brennan JJ (2005). "Combined esterified estrogens and methyltestosterone versus esterified estrogens alone in the treatment of loss of sexual interest in surgically menopausal women". Menopause. 12 (4): 374–84. doi:10.1097/01.GME.0000153933.50860.FD. PMID 16037752.
^ Heiman JR, Rupp H, Janssen E, Newhouse SK, Brauer M, Laan E (May 2011). "Sexual desire, sexual arousal and hormonal differences in premenopausal US and Dutch women with and without low sexual desire". Hormones and Behavior. 59 (5): 772–9. doi:10.1016/j.yhbeh.2011.03.013. PMID 21514299.
^ Brisken C, O'Malley B (December 2010). "Hormone action in the mammary gland". Cold Spring Harbor Perspectives in Biology. 2 (12): a003178. doi:10.1101/cshperspect.a003178. PMC 2982168. PMID 20739412.
^ Kleinberg DL (February 1998). "Role of IGF-I in normal mammary development". Breast Cancer Research and Treatment. 47 (3): 201–8. doi:10.1023/a:1005998832636. PMID 9516076.
^ abc Johnson LR (2003). Essential Medical Physiology. Academic Press. p. 770. ISBN 978-0-12-387584-6.
^ abc Norman AW, Henry HL (30 July 2014). Hormones. Academic Press. p. 311. ISBN 978-0-08-091906-5.
^ ab Coad J, Dunstall M (2011). Anatomy and Physiology for Midwives, with Pageburst online access,3: Anatomy and Physiology for Midwives. Elsevier Health Sciences. p. 413. ISBN 978-0-7020-3489-3.
^ Haslam SZ, Osuch JR (1 January 2006). Hormones and Breast Cancer in Post-Menopausal Women. IOS Press. p. 69. ISBN 978-1-58603-653-9.
^ Silbernagl S, Despopoulos A (1 January 2011). Color Atlas of Physiology. Thieme. pp. 305–. ISBN 978-3-13-149521-1.
^ Fadem B (2007). High-yield Comprehensive USMLE Step 1 Review. Lippincott Williams & Wilkins. pp. 445–. ISBN 978-0-7817-7427-7.
^ Blackburn S (14 April 2014). Maternal, Fetal, & Neonatal Physiology. Elsevier Health Sciences. pp. 146–. ISBN 978-0-323-29296-2.
^ Strauss JF, Barbieri RL (13 September 2013). Yen and Jaffe's Reproductive Endocrinology. Elsevier Health Sciences. pp. 236–. ISBN 978-1-4557-2758-2.
^ Wilson CB, Nizet V, Maldonado Y, Remington JS, Klein JO (24 February 2015). Remington and Klein's Infectious Diseases of the Fetus and Newborn Infant. Elsevier Health Sciences. pp. 190–. ISBN 978-0-323-24147-2.
^ Sherwin BB (February 2012). "Estrogen and cognitive functioning in women: lessons we have learned". Behavioral Neuroscience. 126 (1): 123–7. doi:10.1037/a0025539. PMC 4838456. PMID 22004260.
^ Hara Y, Waters EM, McEwen BS, Morrison JH (July 2015). "Estrogen Effects on Cognitive and Synaptic Health Over the Lifecourse". Physiological Reviews. 95 (3): 785–807. doi:10.1152/physrev.00036.2014. PMC 4491541. PMID 26109339.
^ Korol DL, Pisani SL (August 2015). "Estrogens and cognition: Friends or foes?: An evaluation of the opposing effects of estrogens on learning and memory". Hormones and Behavior. 74: 105–15. doi:10.1016/j.yhbeh.2015.06.017. PMC 4573330. PMID 26149525.
^ Au A, Feher A, McPhee L, Jessa A, Oh S, Einstein G (January 2016). "Estrogens, inflammation and cognition". Frontiers in Neuroendocrinology. 40: 87–100. doi:10.1016/j.yfrne.2016.01.002. PMID 26774208.
^ Jacobs E, D'Esposito M (April 2011). "Estrogen shapes dopamine-dependent cognitive processes: implications for women's health". The Journal of Neuroscience. 31 (14): 5286–93. doi:10.1523/JNEUROSCI.6394-10.2011. PMC 3089976. PMID 21471363.
^ Colzato LS, Hommel B (1 January 2014). "Effects of estrogen on higher-order cognitive functions in unstressed human females may depend on individual variation in dopamine baseline levels". Frontiers in Neuroscience. 8: 65. doi:10.3389/fnins.2014.00065. PMC 3985021. PMID 24778605.
^ Douma SL, Husband C, O'Donnell ME, Barwin BN, Woodend AK (2005). "Estrogen-related mood disorders: reproductive life cycle factors". ANS. Advances in Nursing Science. 28 (4): 364–75. doi:10.1097/00012272-200510000-00008. PMID 16292022.
^ Osterlund MK, Witt MR, Gustafsson JA (December 2005). "Estrogen action in mood and neurodegenerative disorders: estrogenic compounds with selective properties-the next generation of therapeutics". Endocrine. 28 (3): 235–42. doi:10.1385/ENDO:28:3:235. PMID 16388113.
^ Lasiuk GC, Hegadoren KM (October 2007). "The effects of estradiol on central serotonergic systems and its relationship to mood in women". Biological Research for Nursing. 9 (2): 147–60. doi:10.1177/1099800407305600. PMID 17909167.
^ Grigoriadis S, Seeman M (July 2002). "The role of estrogen in schizophrenia: Implications for schizophrenia practice guidelines for women". Canadian Journal of Psychiatry. 47 (5): 437–42. doi:10.1176/foc.4.1.134.
^ "PMDD/PMS". The Massachusetts General Hospital Center for Women’s Mental Health. Retrieved 12 January 2019.
^ Hill RA, McInnes KJ, Gong EC, Jones ME, Simpson ER, Boon WC (February 2007). "Estrogen deficient male mice develop compulsive behavior". Biological Psychiatry. 61 (3): 359–66. doi:10.1016/j.biopsych.2006.01.012. PMID 16566897.
^ Benmansour S, Weaver RS, Barton AK, Adeniji OS, Frazer A (April 2012). "Comparison of the effects of estradiol and progesterone on serotonergic function". Biological Psychiatry. 71 (7): 633–41. doi:10.1016/j.biopsych.2011.11.023. PMC 3307822. PMID 22225849.
^ Berg SJ, Wynne-Edwards KE (June 2001). "Changes in testosterone, cortisol, and estradiol levels in men becoming fathers". Mayo Clinic Proceedings. 76 (6): 582–92. doi:10.4065/76.6.582. PMID 11393496.
^ abc Cao X, Xu P, Oyola MG, Xia Y, Yan X, Saito K, Zou F, Wang C, Yang Y, Hinton A, Yan C, Ding H, Zhu L, Yu L, Yang B, Feng Y, Clegg DJ, Khan S, DiMarchi R, Mani SK, Tong Q, Xu Y (October 2014). "Estrogens stimulate serotonin neurons to inhibit binge-like eating in mice". The Journal of Clinical Investigation. 124 (10): 4351–62. doi:10.1172/JCI74726. PMC 4191033. PMID 25157819.
^ Jimerson DC, Lesem MD, Kaye WH, Hegg AP, Brewerton TD (September 1990). "Eating disorders and depression: is there a serotonin connection?". Biological Psychiatry. 28 (5): 443–54. doi:10.1016/0006-3223(90)90412-u. PMID 2207221.
^ Klump KL, Keel PK, Racine SE, Burt SA, Burt AS, Neale M, Sisk CL, Boker S, Hu JY (February 2013). "The interactive effects of estrogen and progesterone on changes in emotional eating across the menstrual cycle". Journal of Abnormal Psychology. 122 (1): 131–7. doi:10.1037/a0029524. PMC 3570621. PMID 22889242.
^ ab Edler C, Lipson SF, Keel PK (January 2007). "Ovarian hormones and binge eating in bulimia nervosa". Psychological Medicine. 37 (1): 131–41. doi:10.1017/S0033291706008956. PMID 17038206.
^ ab Klump KL, Racine SE, Hildebrandt B, Burt SA, Neale M, Sisk CL, Boker S, Keel PK (September 2014). "Ovarian Hormone Influences on Dysregulated Eating: A Comparison of Associations in Women with versus without Binge Episodes". Clinical Psychological Science. 2 (4): 545–559. doi:10.1177/2167702614521794. PMC 4203460. PMID 25343062.
^ ab Klump KL, Keel PK, Culbert KM, Edler C (December 2008). "Ovarian hormones and binge eating: exploring associations in community samples". Psychological Medicine. 38 (12): 1749–57. doi:10.1017/S0033291708002997. PMC 2885896. PMID 18307829.
^ Lester NA, Keel PK, Lipson SF (January 2003). "Symptom fluctuation in bulimia nervosa: relation to menstrual-cycle phase and cortisol levels". Psychological Medicine. 33 (1): 51–60. doi:10.1017/s0033291702006815. PMID 12537036.
^ Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda S, Harada N, Shah NM (October 2009). "Estrogen masculinizes neural pathways and sex-specific behaviors". Cell. 139 (1): 61–72. doi:10.1016/j.cell.2009.07.036. PMC 2851224. PMID 19804754.
^ Rochira V, Carani C (October 2009). "Aromatase deficiency in men: a clinical perspective". Nature Reviews. Endocrinology. 5 (10): 559–68. doi:10.1038/nrendo.2009.176. PMID 19707181.
^ Wilson JD (September 2001). "Androgens, androgen receptors, and male gender role behavior". Hormones and Behavior. 40 (2): 358–66. doi:10.1006/hbeh.2001.1684. PMID 11534997.
^ Baum MJ (November 2006). "Mammalian animal models of psychosexual differentiation: when is 'translation' to the human situation possible?". Hormones and Behavior. 50 (4): 579–88. doi:10.1016/j.yhbeh.2006.06.003. PMID 16876166.
^ Rosano GM, Panina G (1999). "Oestrogens and the heart". Thérapie. 54 (3): 381–5. PMID 10500455.
^ ab Nadkarni S, Cooper D, Brancaleone V, Bena S, Perretti M (November 2011). "Activation of the annexin A1 pathway underlies the protective effects exerted by estrogen in polymorphonuclear leukocytes". Arteriosclerosis, Thrombosis, and Vascular Biology. 31 (11): 2749–59. doi:10.1161/ATVBAHA.111.235176. PMC 3357483. PMID 21836070.
^ Häggström, Mikael; Richfield, David (2014). "Diagram of the pathways of human steroidogenesis". WikiJournal of Medicine. 1 (1). doi:10.15347/wjm/2014.005. ISSN 2002-4436.
^ Marieb E (2013). Anatomy & physiology. Benjamin-Cummings. p. 903. ISBN 978-0-321-88760-3.
^ Hemsell DL, Grodin JM, Brenner PF, Siiteri PK, MacDonald PC (March 1974). "Plasma precursors of estrogen. II. Correlation of the extent of conversion of plasma androstenedione to estrone with age". The Journal of Clinical Endocrinology and Metabolism. 38 (3): 476–9. doi:10.1210/jcem-38-3-476. PMID 4815174.
^ Barakat R, Oakley O, Kim H, Jin J, Ko CJ (September 2016). "Extra-gonadal sites of estrogen biosynthesis and function". BMB Reports. 49 (9): 488–96. doi:10.5483/BMBRep.2016.49.9.141. PMC 5227141. PMID 27530684.
^ ab Nelson LR, Bulun SE (September 2001). "Estrogen production and action". Journal of the American Academy of Dermatology. 45 (3 Suppl): S116–24. doi:10.1067/mjd.2001.117432. PMID 11511861.
^ Labrie F, Bélanger A, Luu-The V, Labrie C, Simard J, Cusan L, Gomez JL, Candas B (1998). "DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: its role during aging". Steroids. 63 (5–6): 322–8. doi:10.1016/S0039-128X(98)00007-5. PMID 9618795.
^ Buchsbaum HJ, ed. (2012). The Menopause (Clinical Perspectives in Obstetrics and Gynecology). New York, NY: Springer Science & Business Media. p. 64. ISBN 9781461255253.
^ Kuhl H (August 2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric : the Journal of the International Menopause Society. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
^ "EC 2.4.1.17 – glucuronosyltransferase and Organism(s) Homo sapiens". BRENDA. Technische Universität Braunschweig. January 2018. Retrieved 10 August 2018.
^ Kuhl, H (2009). "Pharmacology of estrogens and progestogens: Influence of different routes of administration". Climacteric. 8: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
^ Tata, Jamshed R (2005). "One hundred years of hormones". EMBO Reports. 6 (6): 490–6. doi:10.1038/sj.embor.7400444. PMC 1369102. PMID 15940278.
^ ab "Origin in Biomedical Terms: oestrogen or oestrogen". Bioetymology. Retrieved 2018-01-24.
^ "Council on Pharmacy and Chemistry". Journal of the American Medical Association. 107 (15): 1221–3. 1936. doi:10.1001/jama.1936.02770410043011.
^ "Greek Word Study Tool: oistros". Perseus Digital Library. Retrieved 28 December 2011.
^ Fang H, Tong W, Shi LM, Blair R, Perkins R, Branham W, Hass BS, Xie Q, Dial SL, Moland CL, Sheehan DM (March 2001). "Structure-activity relationships for a large diverse set of natural, synthetic, and environmental estrogens". Chemical Research in Toxicology. 14 (3): 280–94. CiteSeerX 10.1.1.460.20. doi:10.1021/tx000208y. PMID 11258977.
^ Wang S, Huang W, Fang G, Zhang Y, Qiao H (2008). "Analysis of steroidal estrogen residues in food and environmental samples". International Journal of Environmental Analytical Chemistry. 88 (1): 1–25. doi:10.1080/03067310701597293.
^ Wise A, O'Brien K, Woodruff T (January 2011). "Are oral contraceptives a significant contributor to the estrogenicity of drinking water?". Environmental Science & Technology. 45 (1): 51–60. doi:10.1021/es1014482. PMID 20977246. Lay summary – Chemical & Engineering News.
^ Liney KE, Jobling S, Shears JA, Simpson P, Tyler CR (October 2005). "Assessing the sensitivity of different life stages for sexual disruption in roach (Rutilus rutilus) exposed to effluents from wastewater treatment works". Environmental Health Perspectives. 113 (10): 1299–307. doi:10.1289/ehp.7921. PMC 1281270. PMID 16203238.
^ Jobling S, Williams R, Johnson A, Taylor A, Gross-Sorokin M, Nolan M, Tyler CR, van Aerle R, Santos E, Brighty G (April 2006). "Predicted exposures to steroid estrogens in U.K. rivers correlate with widespread sexual disruption in wild fish populations". Environmental Health Perspectives. 114 Suppl 1 (Suppl 1): 32–9. doi:10.1289/ehp.8050. PMC 1874167. PMID 16818244.
^ Sanghavi DM (17 October 2006). "Preschool Puberty, and a Search for the Causes". The New York Times. Retrieved 4 June 2008.
^ ab FDA (February 1995). "Products containing estrogenic hormones, placental extract or vitamins". Guide to Inspections of Cosmetic Product Manufacturers. Archived from the original on 14 October 2007. Retrieved 24 October 2006.
External links[edit]
- Nussey and Whitehead: Endocrinology, an integrated approach, Taylor and Francis 2001. Free online textbook.
Categories:
- Alcohols
- Antigonadotropins
- Estranes
- Estrogens
- Fertility
- Hepatotoxins
- Hormones of the hypothalamus-pituitary-gonad axis
- Hormones of the hypothalamic-pituitary-prolactin axis
- Hormones of the ovary
- Hormones of the pregnant female
- Human female endocrine system
- Human hormones
- Mammal female reproductive system
- Prolactin releasers
- Sex hormones
- 1929 in science
- 1929 in Germany
(window.RLQ=window.RLQ||).push(function()mw.config.set("wgPageParseReport":"limitreport":"cputime":"1.848","walltime":"2.087","ppvisitednodes":"value":7834,"limit":1000000,"ppgeneratednodes":"value":0,"limit":1500000,"postexpandincludesize":"value":446267,"limit":2097152,"templateargumentsize":"value":13755,"limit":2097152,"expansiondepth":"value":12,"limit":40,"expensivefunctioncount":"value":18,"limit":500,"unstrip-depth":"value":1,"limit":20,"unstrip-size":"value":288724,"limit":5000000,"entityaccesscount":"value":5,"limit":400,"timingprofile":["100.00% 1569.030 1 -total"," 58.99% 925.573 1 Template:Reflist"," 43.22% 678.066 64 Template:Cite_journal"," 11.19% 175.520 1 Template:Lang"," 8.73% 136.993 16 Template:Navbox"," 4.54% 71.276 1 Template:Infobox_drug_class"," 4.48% 70.347 13 Template:Cite_book"," 4.30% 67.475 1 Template:Infobox"," 3.63% 56.994 2 Template:Annotated_image_4"," 3.31% 51.918 1 Template:Estradiol_metabolism"],"scribunto":"limitreport-timeusage":"value":"1.029","limit":"10.000","limitreport-memusage":"value":23835923,"limit":52428800,"cachereport":"origin":"mw1253","timestamp":"20190408160302","ttl":2592000,"transientcontent":false);mw.config.set("wgBackendResponseTime":2262,"wgHostname":"mw1253"););