Dynorphin A
[dummy-text]
Dynorphin A
Jump to navigation
Jump to search
 | |
| Names | |
|---|---|
| Other names Dynorphin 1-13 | |
| Identifiers | |
CAS Number |
|
PubChem CID |
|
| Properties | |
Chemical formula | C75H126N24O15 |
Molar mass | 1603.95474 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Infobox references | |
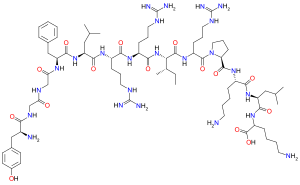
Dynorphin A is a form of dynorphin and an endogenous opioid peptide with the amino acid sequence: Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Arg-Pro-Lys-Leu-Lys.
Dynorphin A1–8 is a truncated form of dynorphin A with the amino acid sequence: Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile.[2][3] Dynorphin A1–8 is an agonist at the mu-, kappa-, and delta-opioid receptors;[3] it has the highest binding affinity for the kappa-opioid receptor.[3]
References[edit]
^ Dynorphin 1-13 - Compound Summary, PubChem.
^ "Dynorphin A 1-8". HMDB Version 4.0. Human Metabolome Database. 27 September 2017. Retrieved 20 October 2017..mw-parser-output cite.citationfont-style:inherit.mw-parser-output .citation qquotes:"""""""'""'".mw-parser-output .citation .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-ws-icon abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center.mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-maintdisplay:none;color:#33aa33;margin-left:0.3em.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ abc "Dynorphin A-(1-8): Biological activity". IUPHAR/BPS Guide to PHARMACOLOGY. International Union of Basic and Clinical Pharmacology. Retrieved 20 October 2017.Principal endogenous agonists at κ receptor
This article about an organic compound is a stub. You can help Wikipedia by expanding it. |
Categories:
- Neuropeptides
- Kappa agonists
- Opioid peptides
- Organic compound stubs
(window.RLQ=window.RLQ||).push(function()mw.config.set("wgPageParseReport":"limitreport":"cputime":"0.792","walltime":"1.003","ppvisitednodes":"value":4356,"limit":1000000,"ppgeneratednodes":"value":0,"limit":1500000,"postexpandincludesize":"value":114108,"limit":2097152,"templateargumentsize":"value":5923,"limit":2097152,"expansiondepth":"value":20,"limit":40,"expensivefunctioncount":"value":1,"limit":500,"unstrip-depth":"value":1,"limit":20,"unstrip-size":"value":7232,"limit":5000000,"entityaccesscount":"value":1,"limit":400,"timingprofile":["100.00% 768.087 1 -total"," 69.59% 534.500 1 Template:Chembox"," 42.30% 324.882 1 Template:Chembox_Identifiers"," 28.30% 217.343 3 Template:Chembox_headerbar"," 27.74% 213.049 9 Template:Trim"," 18.69% 143.542 1 Template:Reflist"," 15.90% 122.097 5 Template:Main_other"," 15.06% 115.649 1 Template:Cite_encyclopedia"," 13.71% 105.315 1 Template:Chembox_parametercheck"," 10.59% 81.304 1 Template:Chembox_Properties"],"scribunto":"limitreport-timeusage":"value":"0.281","limit":"10.000","limitreport-memusage":"value":3747628,"limit":52428800,"cachereport":"origin":"mw1238","timestamp":"20190126191215","ttl":2073600,"transientcontent":false););"@context":"https://schema.org","@type":"Article","name":"Dynorphin A","url":"https://en.wikipedia.org/wiki/Dynorphin_A","sameAs":"http://www.wikidata.org/entity/Q5319233","mainEntity":"http://www.wikidata.org/entity/Q5319233","author":"@type":"Organization","name":"Contributors to Wikimedia projects","publisher":"@type":"Organization","name":"Wikimedia Foundation, Inc.","logo":"@type":"ImageObject","url":"https://www.wikimedia.org/static/images/wmf-hor-googpub.png","datePublished":"2007-09-29T21:20:31Z","dateModified":"2019-01-13T23:18:54Z","image":"https://upload.wikimedia.org/wikipedia/commons/c/cf/Dynorphin_A.svg","headline":"chemical compound"(window.RLQ=window.RLQ||).push(function()mw.config.set("wgBackendResponseTime":1141,"wgHostname":"mw1238"););

