Half-life

 Clash Royale CLAN TAG#URR8PPP
Clash Royale CLAN TAG#URR8PPP
| Number of half-lives elapsed | Fraction remaining | Percentage remaining | |
|---|---|---|---|
| 0 | 1⁄1 | 100 | |
| 1 | 1⁄2 | 50 | |
| 2 | 1⁄4 | 25 | |
| 3 | 1⁄8 | 12 | .5 |
| 4 | 1⁄16 | 6 | .25 |
| 5 | 1⁄32 | 3 | .125 |
| 6 | 1⁄64 | 1 | .563 |
| 7 | 1⁄128 | 0 | .781 |
| ... | ... | ... | |
| n | 1/2n | 100/2n | |
Half-life (symbol t1⁄2) is the time required for a quantity to reduce to half its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo, or how long stable atoms survive, radioactive decay. The term is also used more generally to characterize any type of exponential or non-exponential decay. For example, the medical sciences refer to the biological half-life of drugs and other chemicals in the human body. The converse of half-life is doubling time.
The original term, half-life period, dating to Ernest Rutherford's discovery of the principle in 1907, was shortened to half-life in the early 1950s.[1] Rutherford applied the principle of a radioactive element's half-life to studies of age determination of rocks by measuring the decay period of radium to lead-206.
Half-life is constant over the lifetime of an exponentially decaying quantity, and it is a characteristic unit for the exponential decay equation. The accompanying table shows the reduction of a quantity as a function of the number of half-lives elapsed.
Contents
1 Probabilistic nature
2 Formulas for half-life in exponential decay
2.1 Decay by two or more processes
2.2 Examples
3 In non-exponential decay
4 In biology and pharmacology
5 See also
6 References
7 External links
Probabilistic nature

Simulation of many identical atoms undergoing radioactive decay, starting with either 4 atoms per box (left) or 400 (right). The number at the top is how many half-lives have elapsed. Note the consequence of the law of large numbers: with more atoms, the overall decay is more regular and more predictable.
A half-life usually describes the decay of discrete entities, such as radioactive atoms. In that case, it does not work to use the definition that states "half-life is the time required for exactly half of the entities to decay". For example, if there is just one radioactive atom, and its half-life is one second, there will not be "half of an atom" left after one second.
Instead, the half-life is defined in terms of probability: "Half-life is the time required for exactly half of the entities to decay on average". In other words, the probability of a radioactive atom decaying within its half-life is 50%.
For example, the image on the right is a simulation of many identical atoms undergoing radioactive decay. Note that after one half-life there are not exactly one-half of the atoms remaining, only approximately, because of the random variation in the process. Nevertheless, when there are many identical atoms decaying (right boxes), the law of large numbers suggests that it is a very good approximation to say that half of the atoms remain after one half-life.
There are various simple exercises that demonstrate probabilistic decay, for example involving flipping coins or running a statistical computer program.[2][3][4]
Formulas for half-life in exponential decay
An exponential decay can be described by any of the following three equivalent formulas:
where
N0 is the initial quantity of the substance that will decay (this quantity may be measured in grams, moles, number of atoms, etc.),
N(t) is the quantity that still remains and has not yet decayed after a time t,
t1⁄2 is the half-life of the decaying quantity,
τ is a positive number called the mean lifetime of the decaying quantity,
λ is a positive number called the decay constant of the decaying quantity.
The three parameters t1⁄2, τ, and λ are all directly related in the following way:
where ln(2) is the natural logarithm of 2 (approximately 0.693).
Decay by two or more processes
Some quantities decay by two exponential-decay processes simultaneously. In this case, the actual half-life T1⁄2 can be related to the half-lives t1 and t2 that the quantity would have if each of the decay processes acted in isolation:
- 1T1/2=1t1+1t2displaystyle frac 1T_1/2=frac 1t_1+frac 1t_2
For three or more processes, the analogous formula is:
- 1T1/2=1t1+1t2+1t3+⋯displaystyle frac 1T_1/2=frac 1t_1+frac 1t_2+frac 1t_3+cdots
For a proof of these formulas, see Exponential decay § Decay by two or more processes.
Examples
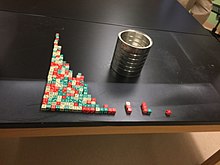
Half life demonstrated using dice in a classroom experiment
There is a half-life describing any exponential-decay process. For example:
- As noted above, in radioactive decay the half-life is the length of time after which there is a 50% chance that an atom will have undergone nuclear decay. It varies depending on the atom type and isotope, and is usually determined experimentally. See List of nuclides.
- The current flowing through an RC circuit or RL circuit decays with a half-life of ln(2)RC or ln(2)L/R, respectively. For this example the term half time tends to be used, rather than "half life", but they mean the same thing.
- In a chemical reaction, the half-life of a species is the time it takes for the concentration of that substance to fall to half of its initial value. In a first-order reaction the half-life of the reactant is ln(2)/λ, where λ is the reaction rate constant.
In non-exponential decay
The term "half-life" is almost exclusively used for decay processes that are exponential (such as radioactive decay or the other examples above), or approximately exponential (such as biological half-life discussed below). In a decay process that is not even close to exponential, the half-life will change dramatically while the decay is happening. In this situation it is generally uncommon to talk about half-life in the first place, but sometimes people will describe the decay in terms of its "first half-life", "second half-life", etc., where the first half-life is defined as the time required for decay from the initial value to 50%, the second half-life is from 50% to 25%, and so on.[5]
In biology and pharmacology
A biological half-life or elimination half-life is the time it takes for a substance (drug, radioactive nuclide, or other) to lose one-half of its pharmacologic, physiologic, or radiological activity. In a medical context, the half-life may also describe the time that it takes for the concentration of a substance in blood plasma to reach one-half of its steady-state value (the "plasma half-life").
The relationship between the biological and plasma half-lives of a substance can be complex, due to factors including accumulation in tissues, active metabolites, and receptor interactions.[6]
While a radioactive isotope decays almost perfectly according to so-called "first order kinetics" where the rate constant is a fixed number, the elimination of a substance from a living organism usually follows more complex chemical kinetics.
For example, the biological half-life of water in a human being is about 9 to 10 days,[7] though this can be altered by behavior and various other conditions. The biological half-life of caesium in human beings is between one and four months.
The concept of a half-life has also been utilized for pesticides in plants,[8] and certain authors maintain that pesticide risk and impact assessment models rely on and are sensitive to information describing dissipation from plants.[9]
See also
- Half time (physics)
- List of isotopes by half-life
- Mean lifetime
References
^ John Ayto, 20th Century Words (1989), Cambridge University Press.
^ Chivers, Sidney (March 16, 2003). "Re: What happens durring half lifes [sic] when there is only one atom left?". MADSCI.org..mw-parser-output cite.citationfont-style:inherit.mw-parser-output .citation qquotes:"""""""'""'".mw-parser-output .citation .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-ws-icon abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center.mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-maintdisplay:none;color:#33aa33;margin-left:0.3em.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^
"Radioactive-Decay Model". Exploratorium.edu. Retrieved 2012-04-25.
^
Wallin, John (September 1996). "Assignment #2: Data, Simulations, and Analytic Science in Decay". Astro.GLU.edu. Archived from the original on 2011-09-29.CS1 maint: BOT: original-url status unknown (link)
^ Jonathan Crowe, Tony Bradshaw (2014). Chemistry for the Biosciences: The Essential Concepts. p. 568. ISBN 9780199662883.CS1 maint: Uses authors parameter (link)
^ Lin VW; Cardenas DD (2003). Spinal cord medicine. Demos Medical Publishing, LLC. p. 251. ISBN 978-1-888799-61-3.
^ Pang, Xiao-Feng (2014). Water: Molecular Structure and Properties. New Jersey: World Scientific. p. 451. ISBN 9789814440424.
^ Australian Pesticides and Veterinary Medicines Authority (31 March 2015). "Tebufenozide in the product Mimic 700 WP Insecticide, Mimic 240 SC Insecticide". Australian Government. Retrieved 30 April 2018.
^ Fantke, Peter; Gillespie, Brenda W.; Juraske, Ronnie; Jolliet, Olivier (11 July 2014). "Estimating Half-Lives for Pesticide Dissipation from Plants". Environmental Science & Technology. 48 (15): 8588–8602. Bibcode:2014EnST...48.8588F. doi:10.1021/es500434p. PMID 24968074.
External links
| Look up half-life in Wiktionary, the free dictionary. |
| Wikimedia Commons has media related to Half times. |
Nucleonica.net, Nuclear Science Portal
Nucleonica.net, wiki: Decay Engine
Bucknell.edu, System Dynamics – Time Constants
Subotex.com, Half-Life elimination of drugs in blood plasma – Simple Charting Tool



