Vincamine
Vincamine
Jump to navigation
Jump to search
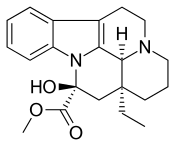 | |
| Clinical data | |
|---|---|
| Trade names | Oxybral SR |
AHFS/Drugs.com | International Drug Names |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
PubChem CID |
|
| IUPHAR/BPS |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEMBL |
|
| ECHA InfoCard | 100.015.070 |
| Chemical and physical data | |
| Formula | C21H26N2O3 |
| Molar mass | 7002354450000000000♠354.450 g·mol−1 |
| 3D model (JSmol) |
|
SMILES
| |
InChI
| |
.mw-parser-output .noboldfont-weight:normal (verify) | |
Vincamine is a nootropic monoterpenoid indole alkaloid found in the leaves of Vinca minor (lesser periwinkle), comprising about 25-65% of its indole alkaloids by weight. It can also be synthesized from related alkaloids.[1]
It is related to the nootropic vinpocetine. [2]
Contents
1 Uses
2 See also
3 References
4 External links
Uses[edit]
Vincamine is sold in Europe as a memory enhancing prescription medicine for the treatment of primary degenerative and vascular dementia., in the US as a dietary supplement approved by the FDA for uses of 6 months or less, and as a generic drugs in some regions.[3] Most common preparations are in the sustained release tablet forms.
See also[edit]
- Vinpocetine
References[edit]
^ "Indole Alkaloids". Ullmann's Encyclopedia of Industrial Chemistry (Fifth ed.). Wiley-VCH. 1985. p. 393. ISBN 3-527-20100-9..mw-parser-output cite.citationfont-style:inherit.mw-parser-output .citation qquotes:"""""""'""'".mw-parser-output .citation .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-ws-icon abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center.mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-maintdisplay:none;color:#33aa33;margin-left:0.3em.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ https://www.fda.gov/Food/DietarySupplements/ProductsIngredients/ucm518478.htm
^ https://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/vincamine_508.pdf
External links[edit]
"Vincamine MSDS" (pdf).
[dead link]
Chemical Selection Working Group. "Vincamine - 1617-90-9" (pdf). Summary of Data for Chemical Selection. NIH - United States National Institutes of Health. Archived (PDF) from the original on 2011-10-21. Retrieved 2007-04-23.
Categories:
- Tertiary alcohols
- Carboxylate esters
- Methyl esters
- Nitrogen heterocycles
- Vinca alkaloids
- Heterocyclic compounds (4 or more rings)
(window.RLQ=window.RLQ||).push(function()mw.config.set("wgPageParseReport":"limitreport":"cputime":"0.592","walltime":"0.751","ppvisitednodes":"value":4815,"limit":1000000,"ppgeneratednodes":"value":0,"limit":1500000,"postexpandincludesize":"value":71705,"limit":2097152,"templateargumentsize":"value":6511,"limit":2097152,"expansiondepth":"value":18,"limit":40,"expensivefunctioncount":"value":2,"limit":500,"unstrip-depth":"value":1,"limit":20,"unstrip-size":"value":7801,"limit":5000000,"entityaccesscount":"value":1,"limit":400,"timingprofile":["100.00% 685.492 1 -total"," 76.95% 527.507 1 Template:Drugbox"," 55.37% 379.575 1 Template:Infobox"," 13.99% 95.880 16 Template:Unbulleted_list"," 13.07% 89.599 1 Template:Reflist"," 11.95% 81.929 1 Template:Chem_molar_mass"," 11.60% 79.524 1 Template:Cite_encyclopedia"," 9.60% 65.821 1 Template:Chem_molar_mass/format"," 8.29% 56.818 1 Template:Val"," 5.48% 37.586 1 Template:Dead_link"],"scribunto":"limitreport-timeusage":"value":"0.251","limit":"10.000","limitreport-memusage":"value":5651979,"limit":52428800,"cachereport":"origin":"mw1335","timestamp":"20190329175758","ttl":2592000,"transientcontent":false);mw.config.set("wgBackendResponseTime":92,"wgHostname":"mw1249"););

