Genetic disorder
| Genetic disorder | |
|---|---|
 | |
| A boy with Down syndrome, one of the most common genetic disorders | |
| Specialty | Medical genetics |
A genetic disorder is a genetic problem caused by one or more abnormalities formed in the genome. Most genetic disorders are quite rare and affect one person in every several thousands or millions.[citation needed] The earliest known genetic condition in a hominid was in the fossil species Paranthropus robustus, with over a third of individuals displaying Amelogenesis imperfecta.[1]
Genetic disorders may be hereditary, meaning that they are passed down from the parents' genes. In other genetic disorders, defects may be caused by new mutations or changes to the DNA. In such cases, the defect will only be passed down if it occurs in the germline.
Some types of recessive gene disorders confer an advantage in certain environments when only one copy of the gene is present.[2]
Contents
1 Single-gene
1.1 Autosomal dominant
1.2 Autosomal recessive
1.3 X-linked dominant
1.4 X-linked recessive
1.5 Y-linked
1.6 Mitochondrial
2 Multiple genes
3 Diagnosis
4 Prognosis
5 Treatment
6 See also
7 References
8 External links
Single-gene
| Disorder prevalence (approximate) | |
|---|---|
| Autosomal dominant | |
| Familial hypercholesterolemia | 1 in 500 |
| Polycystic kidney disease | 1 in 1250 |
| Neurofibromatosis type I | 1 in 2,500 |
| Hereditary spherocytosis | 1 in 5,000 |
| Marfan syndrome | 1 in 4,000[3] |
| Huntington's disease | 1 in 15,000[4] |
| Autosomal recessives | |
| Sickle cell anaemia | 1 in 625 |
| Cystic fibrosis | 1 in 2,000 |
| Tay–Sachs disease | 1 in 3,000 |
| Phenylketonuria | 1 in 12,000 |
| Mucopolysaccharidoses | 1 in 25,000 |
| Lysosomal acid lipase deficiency | 1 in 40,000 |
| Glycogen storage diseases | 1 in 50,000 |
| Galactosemia | 1 in 57,000 |
| X-linked | |
| Duchenne muscular dystrophy | 1 in 7,000 |
| Hemophilia | 1 in 10,000 |
| Values are for liveborn infants | |
A single-gene (or monogenic) disorder is the result of a single mutated gene. Over 6000 human diseases are caused by single-gene defects.[5] Single-gene disorders can be passed on to subsequent generations in several ways. Genomic imprinting and uniparental disomy, however, may affect inheritance patterns. The divisions between recessive and dominant types are not "hard and fast", although the divisions between autosomal and X-linked types are (since the latter types are distinguished purely based on the chromosomal location of the gene). For example, achondroplasia is typically considered as a dominant disorder, but children with two genes for achondroplasia have a severe skeletal disorder of which achondroplasics could be viewed as carriers. Sickle-cell anemia is also considered as a recessive condition, but heterozygous carriers have increased resistance to malaria in early childhood, which could be described as a related dominant condition.[6] When a couple where one partner or both are sufferers or carriers of a single-gene disorder wish to have a child, they can do so through in vitro fertilization, which enables preimplantation genetic diagnosis to occur to check whether the embryo has the genetic disorder.[7]
Most congenital metabolic disorders known as inborn errors of metabolism result from single-gene defects.
Autosomal dominant
Only one mutated copy of the gene will be necessary for a person to be affected by an autosomal dominant disorder. Each affected person usually has one affected parent.[8] The chance a child will inherit the mutated gene is 50%. Autosomal dominant conditions sometimes have reduced penetrance, which means although only one mutated copy is needed, not all individuals who inherit that mutation go on to develop the disease. Examples of this type of disorder are Huntington's disease,[9]neurofibromatosis type 1, neurofibromatosis type 2, Marfan syndrome, hereditary nonpolyposis colorectal cancer, hereditary multiple exostoses (a highly penetrant autosomal dominant disorder), Tuberous sclerosis, Von Willebrand disease, and acute intermittent porphyria. Birth defects are also called congenital anomalies.
Autosomal recessive
Two copies of the gene must be mutated for a person to be affected by an autosomal recessive disorder. An affected person usually has unaffected parents who each carry a single copy of the mutated gene (and are referred to as carriers). Two unaffected people who each carry one copy of the mutated gene have a 25% risk with each pregnancy of having a child affected by the disorder. Examples of this type of disorder are Albinism, Medium-chain acyl-CoA dehydrogenase deficiency, cystic fibrosis, sickle-cell disease, Tay–Sachs disease, Niemann-Pick disease, spinal muscular atrophy, and Roberts syndrome. Certain other phenotypes, such as wet versus dry earwax, are also determined in an autosomal recessive fashion.[10][11]
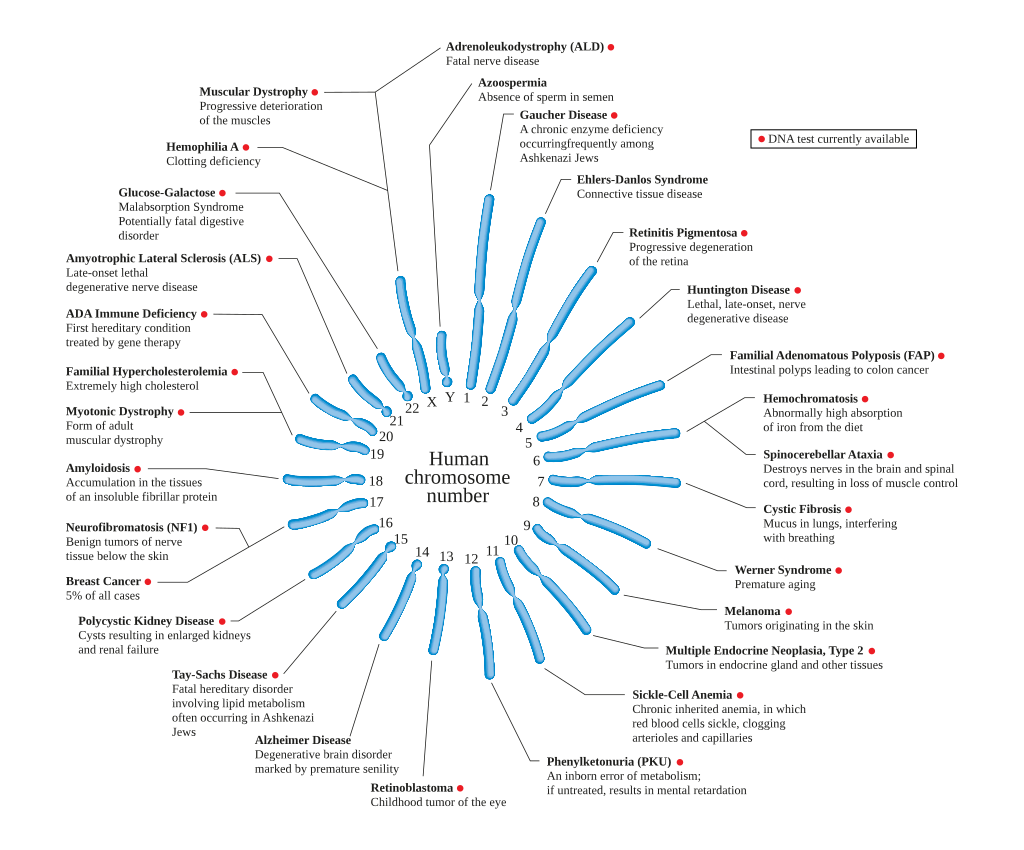
X-linked dominant
X-linked dominant disorders are caused by mutations in genes on the X chromosome. Only a few disorders have this inheritance pattern, with a prime example being X-linked hypophosphatemic rickets. Males and females are both affected in these disorders, with males typically being more severely affected than females. Some X-linked dominant conditions, such as Rett syndrome, incontinentia pigmenti type 2, and Aicardi syndrome, are usually fatal in males either in utero or shortly after birth, and are therefore predominantly seen in females. Exceptions to this finding are extremely rare cases in which boys with Klinefelter syndrome (47,XXY) also inherit an X-linked dominant condition and exhibit symptoms more similar to those of a female in terms of disease severity. The chance of passing on an X-linked dominant disorder differs between men and women. The sons of a man with an X-linked dominant disorder will all be unaffected (since they receive their father's Y chromosome), and his daughters will all inherit the condition. A woman with an X-linked dominant disorder has a 50% chance of having an affected fetus with each pregnancy, although in cases such as incontinentia pigmenti, only female offspring are generally viable.
X-linked recessive
X-linked recessive conditions are also caused by mutations in genes on the X chromosome. Males are more frequently affected than females, and the chance of passing on the disorder differs between men and women. The sons of a man with an X-linked recessive disorder will not be affected, and his daughters will carry one copy of the mutated gene. A woman who is a carrier of an X-linked recessive disorder (XRXr) has a 50% chance of having sons who are affected and a 50% chance of having daughters who carry one copy of the mutated gene and are therefore carriers. X-linked recessive conditions include the serious diseases hemophilia A, Duchenne muscular dystrophy, and Lesch-Nyhan syndrome, as well as common and less serious conditions such as male pattern baldness and red-green color blindness. X-linked recessive conditions can sometimes manifest in females due to skewed X-inactivation or monosomy X (Turner syndrome).
Y-linked
Y-linked disorders are caused by mutations on the Y chromosome. These conditions may only be transmitted from the heterogametic sex (e.g. male humans) to offspring of the same sex. More simply, this means that Y-linked disorders in humans can only be passed from men to their sons; females can never be affected because they do not possess Y-allosomes.
Y-linked disorders are exceedingly rare but the most well-known examples typically cause infertility. Reproduction in such conditions is only possible through the circumvention of infertility by medical intervention.
Mitochondrial
This type of inheritance, also known as maternal inheritance, applies to genes encoded by mitochondrial DNA. Because only egg cells contribute mitochondria to the developing embryo, only mothers can pass on mitochondrial DNA conditions to their children. An example of this type of disorder is Leber's hereditary optic neuropathy. It is important to stress that the vast majority of mitochondrial disease (particularly when symptoms develop in early life) is actually caused by an underlying nuclear gene defect, and most often follows autosomal recessive inheritance.[12]
Multiple genes
Genetic disorders may also be complex, multifactorial, or polygenic, meaning they are likely associated with the effects of multiple genes in combination with lifestyles and environmental factors. Multifactorial disorders include heart disease and diabetes. Although complex disorders often cluster in families, they do not have a clear-cut pattern of inheritance. This makes it difficult to determine a person’s risk of inheriting or passing on these disorders. Complex disorders are also difficult to study and treat, because the specific factors that cause most of these disorders have not yet been identified. Studies which aim to identify the cause of complex disorders can use several methodological approaches to determine genotype-phenotype associations. One method, the genotype-first approach, starts by identifying genetic variants within patients and then determining the associated clinical manifestations. This is opposed to the more traditional phenotype-first approach, and may identify causal factors that have previously been obscured by clinical heterogeneity, penetrance, and expressivity.
On a pedigree, polygenic diseases do tend to "run in families", but the inheritance does not fit simple patterns as with Mendelian diseases. But this does not mean that the genes cannot eventually be located and studied. There is also a strong environmental component to many of them (e.g., blood pressure).
- asthma
autoimmune diseases such as multiple sclerosis- cancers
- ciliopathies
- cleft palate
- diabetes
- heart disease
- hypertension
- inflammatory bowel disease
- intellectual disability
- mood disorder
- obesity
- refractive error
- infertility
Diagnosis
Due to the wide range of genetic disorders that are known, diagnosis is widely varied and dependent of the disorder. Most genetic disorders are diagnosed at birth or during early childhood however some, such as Huntington's disease, can escape detection until the patient is well into adulthood.
The basic aspects of a genetic disorder rests on the inheritance of genetic material. With an in depth family history, it is possible to anticipate possible disorders in children which direct medical professionals to specific tests depending on the disorder and allow parents the chance to prepare for potential lifestyle changes, anticipate the possibility of stillbirth, or contemplate termination.[13]Prenatal diagnosis can detect the presence of characteristic abnormalities in fetal development through ultrasound, or detect the presence of characteristic substances via invasive procedures which involve inserting probes or needles into the uterus such as in amniocentesis.[14]
Prognosis
Not all genetic disorders directly result in death; however, there are no known cures for genetic disorders. Many genetic disorders affect stages of development, such as Down syndrome, while others result in purely physical symptoms such as muscular dystrophy. Other disorders, such as Huntington's disease, show no signs until adulthood. During the active time of a genetic disorder, patients mostly rely on maintaining or slowing the degradation of quality of life and maintain patient autonomy. This includes physical therapy, pain management, and may include a selection of alternative medicine programs.
Treatment

From personal genomics to gene therapy
The treatment of genetic disorders is an ongoing battle with over 1800 gene therapy clinical trials having been completed, are ongoing, or have been approved worldwide.[15] Despite this, most treatment options revolve around treating the symptoms of the disorders in an attempt to improve patient quality of life.
Gene therapy refers to a form of treatment where a healthy gene is introduced to a patient. This should alleviate the defect caused by a faulty gene or slow the progression of disease. A major obstacle has been the delivery of genes to the appropriate cell, tissue, and organ affected by the disorder. How does one introduce a gene into the potentially trillions of cells which carry the defective copy? This question has been the roadblock between understanding the genetic disorder and correcting the genetic disorder.[16]
See also
FINDbase (the Frequency of Inherited Disorders database)- Genetic epidemiology
- List of genetic disorders
- Population groups in biomedicine
- Mendelian error
References
^ "A probable genetic origin for pitting enamel hypoplasia on the molars of Paranthropus robustus | Request PDF". ResearchGate. Retrieved 2019-03-09..mw-parser-output cite.citationfont-style:inherit.mw-parser-output .citation qquotes:"""""""'""'".mw-parser-output .citation .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-ws-icon abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center.mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-maintdisplay:none;color:#33aa33;margin-left:0.3em.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ Mitton, Jeffery, B (2002). "Heterozygous Advantage". eLS. doi:10.10.38/npg.els.0001760 (inactive 2019-03-14).
^ Keane MG; Pyeritz RE (May 2008). "Medical management of Marfan syndrome". Circulation. 117 (21): 2802–13. doi:10.1161/CIRCULATIONAHA.107.693523. PMID 18506019.
^ Walker FO (2007). "Huntington's disease". Lancet. 369 (9557): 218–28 [221]. doi:10.1016/S0140-6736(07)60111-1. PMID 17240289.
^ "Error 403".
^ Williams T. N.; Obaro S. K. (2011). "Sickle cell disease and malaria morbidity: a tale with two tails". Trends in Parasitology. 27 (7): 315–320. doi:10.1016/j.pt.2011.02.004. PMID 21429801.
^ Kuliev A; Verlinsky Y (2005). "Preimplantation diagnosis: A realistic option for assisted reproduction and genetic practice". Curr. Opin. Obstet. Gynecol. 17 (2): 179–83. doi:10.1097/01.gco.0000162189.76349.c5. PMID 15758612. Retrieved 2009-04-01.
^ Griffiths, Anthony J.F.; Wessler, Susan R.; Carroll, Sean B.; Doebley, John (2012). "2: Single-Gene Inheritance". Introduction to Genetic Analysis (10th ed.). New York: W.H. Freeman and Company. p. 57. ISBN 978-1-4292-2943-2.
^ Griffiths, Anthony J.F.; Wessler, Susan R.; Carroll, Sean B.; Doebley, John (2012). Introduction to Genetic Analysis (10th ed.). New York: W.H. Freeman and Company. p. 58. ISBN 978-1-4292-2943-2.
^ Wade, Nicholas (January 29, 2006). "Japanese Scientists Identify Ear Wax Gene". New York Times.
^ Yoshiura K; Kinoshita A; Ishida T; et al. (March 2006). "A SNP in the ABCC11 gene is the determinant of human earwax type". Nat. Genet. 38 (3): 324–30. doi:10.1038/ng1733. PMID 16444273.
^ Robert, Nussbaum; McInnes, Roderick; Willard, Huntington (2007). Thompson & Thompson Genetics in Medicine. Philadelphia PA: Saunders. pp. 144, 145, 146. ISBN 9781416030805.
^ Milunsky, edited by Aubrey (2004). Genetic disorders and the fetus : diagnosis, prevention, and treatment (5th ed.). Baltimore: Johns Hopkins University Press. ISBN 978-0801879289.CS1 maint: Extra text: authors list (link)
^ "Diagnostic Tests – Amniocentesis". Harvard Medical School. Archived from the original on 2008-05-16. Retrieved 2008-07-15.
^ Ginn, Samantha L.; Alexander, Ian E.; Edelstein, Michael L.; Abedi, Mohammad R.; Wixon, Jo (February 2013). "Gene therapy clinical trials worldwide to 2012 – an update". The Journal of Gene Medicine. 15 (2): 65–77. doi:10.1002/jgm.2698. PMID 23355455.
^ Verma, I. M. (22 August 2013). "Gene Therapy That Works". Science. 341 (6148): 853–855. doi:10.1126/science.1242551. PMID 23970689.
External links
| Classification | D
|
|---|
- Public Health Genomics at CDC
- OMIM — Online Mendelian Inheritance in Man, a catalog of human genes and genetic disorders
Genetic and Rare Diseases Information Center (GARD) Office of Rare Diseases (ORD), National Institutes of Health (NIH)- CDC’s National Center on Birth Defects and Developmental Disabilities
- Genetic Disease Information from the Human Genome Project
- Global Genes Project, Genetic and Rare Diseases Organization